SUMMARY
Blue mould [Peronospora hyoscyami f. sp. tabacina (Adam) Skalicky 1964] is one of the most important foliar diseases of tobacco that causes significant losses in the Americas, south‐eastern Europe and the Middle East. This review summarizes the current knowledge of the mechanisms employed by this oomycete pathogen to colonize its host, with emphasis on molecular aspects of pathogenicity. In addition, key biochemical and molecular mechanisms involved in tobacco resistance to blue mould are discussed.
Taxonomy: Kingdom: Chromista (Straminipila); Phylum: Heterokontophyta; Class: Oomycete; Order: Peronosporales; Family: Peronosporaceae; Genus: Peronospora; Species: Peronospora hyoscyami f. sp. tabacina.
Disease symptoms: The pathogen typically causes localized lesions on tobacco leaves that appear as single, or groups of, yellow spots that often coalesce to form light‐brown necrotic areas. Some of the leaves exhibit grey to bluish downy mould on their lower surfaces. Diseased leaves can become twisted, such that the lower surfaces turn upwards. In such cases, the bluish colour of the diseased plants becomes quite conspicuous, especially under moist conditions when sporulation is abundant. Hence the name of the disease: tobacco blue mould.
Infection process: The pathogen develops haustoria within plant cells that are thought to establish the transfer of nutrients from the host cell, and may also act in the delivery of effector proteins during infection.
Resistance: Several defence responses have been reported to occur in the Nicotiana tabacum–P. hyoscyami f. sp. tabacina interaction. These include the induction of pathogenesis‐related genes, and a correlated increase in the activities of typical pathogenesis‐related proteins, such as peroxidases, chitinases, β‐1,3‐glucanases and lipoxygenases. Systemic acquired resistance is one of the best characterized tobacco defence responses activated on pathogen infection.
INTRODUCTION
Blue mould (Peronospora hyoscyami f. sp. tabacina) is one of the most important foliar diseases of tobacco that causes significant losses in the Americas, south‐eastern Europe and the Middle East. It was first reported in tobacco‐growing areas of Australia during the 1800s (Cooke, 1891). Blue mould epidemics have resulted in annual losses exceeding $200 million in North America (Heist et al., 2002; Lucas, 1980; Nesmith, 1984). In Cuba, the disease caused severe losses between 1978 and 1980 (Pérez et al., 2003).
The development of biotechnological tools has permitted the identification of tobacco genes that are involved in resistance against blue mould (Alexander et al., 1993; Borrás‐Hidalgo et al., 2006; Kroumova et al., 2007; Lusso and Kuc, 1996; Salt et al., 1986; Schiltz, 1974). Furthermore, the identification of molecular markers linked to genetic factors controlling resistance has been improved (Julio et al., 2006; Milla et al., 2005). This may also facilitate the development of resistant cultivars. This review summarizes the current knowledge of the pathogenicity mechanisms employed by the oomycete to colonize its host. In addition, the biochemistry and molecular events involved in tobacco resistance to blue mould are discussed.
SYMPTOMS OF TOBACCO BLUE MOULD DISEASE
The pathogen is capable of infecting tobacco plants in growing regions worldwide throughout the growing season (including transplant production) and can spread rapidly under favourable weather conditions (Main, 1991). If the weather is cloudy and cool, the disease can result in complete crop destruction (Lucas, 1975). On seedbeds of small seedlings with leaves of less than 2 cm in diameter, small patches of dead or dying seedlings with erect leaves provide evidence of the disease (Lucas, 1975; Wolf et al., 1934). After 7–10 days, when sufficient secondary inoculum has been produced, a general epidemic occurs and the entire seedbed may be affected. Older plants with leaves up to 4 cm exhibit a grey or bluish downy mould on the lower surface (Fig. 1). The upper surfaces of infected leaves can remain almost normal in appearance for 1–2 days before the plants begin to die and turn light brown, especially under wet conditions, where sporulation is abundant. In addition, vascular discoloration inside the stems caused by systemic stem infection may occur, resulting in partial or overall stunting of the plant (Lucas, 1975; Reuveni et al., 1986). If this occurs near the base of stems, the plants often lodge or snap off.
Figure 1.

Symptoms and conidiophores of Peronospora hyoscyami f. sp. tabacina: (A) leaf lesions on field tobacco produced by tobacco blue mould; (B) typical symptoms of blue mould; (C) P. hyoscyami f. sp. tabacina sporulation on the lower side of the leaf; (D) microscopic observation of conidiophores produced by P. hyoscyami f. sp. tabacina.
CAUSAL ORGANISM AND DISEASE CYCLE
Oomycetes are traditionally treated within mycology; however, ultrastructural, biochemical and molecular phylogenetic data demonstrate that they are not related to true fungi (Kingdom Fungi), but belong to the Kingdom Chromista (Straminipila), which also contains the chromistan (heterokont) algae (Dick, 2002; Kirk et al., 2001; Voglmayr, 2008).
The blue mould pathogen belongs to the Class Oomycetes, Order Peronosporales and Family Peronosporaceae (Voglmayr, 2008). Molecular phylogenies using internal transcribed spacer (ITS) data not only confirm a rather narrow species concept in Peronospora, but also help to clarify species attribution (Voglmayr, 2008). This downy mildew pathogen of the Solanaceae was originally described from a Hyoscyamus sp. in Czechoslovakia in 1859, as Peronospora effusa var. hyoscyami. In 1863, de Bary elevated it to the rank of species, as P. hyoscyami de Bary 1863. In 1933, Adam proposed the name P. tabacina for the blue mould of Nicotiana tabacum (tobacco) and other Nicotiana spp., but acknowledged that there were few morphological differences from P. hyoscyami. Analysis of a wider sample of isolates revealed no consistent morphological differences between P. hyoscyami and P. tabacina, and therefore P. tabacina was considered to be a synonym of the earlier name P. hyoscyami (Shepherd, 1970). Nevertheless, the name P. tabacina has continued to appear in the literature (Schiltz, 1981; Voglmayr, 2008).
The pathogen is an obligate biotrophic parasite and produces both conidiophores and oospores (Fig. 2). The hyaline and lemon‐shaped conidiophores (15 × 25 µm) are borne on tree‐like and dichotomously branched, which terminate in curved, acute apices (Fig. 1D). The conidiophores emerge through the stomates on the underside of the leaf in great numbers and vary from 400 to 750 µm in length (Fig. 1D). The conidiophores are fragile and short‐lived. They are sensitive to UV light and, when released, exposure to direct sunlight kills most within 1 h (Aylor, 1986; Bashi and Aylor, 1983; Rotem et al., 1985). They seldom are found viable on leaf lesions more than 72–96 h old.
Figure 2.
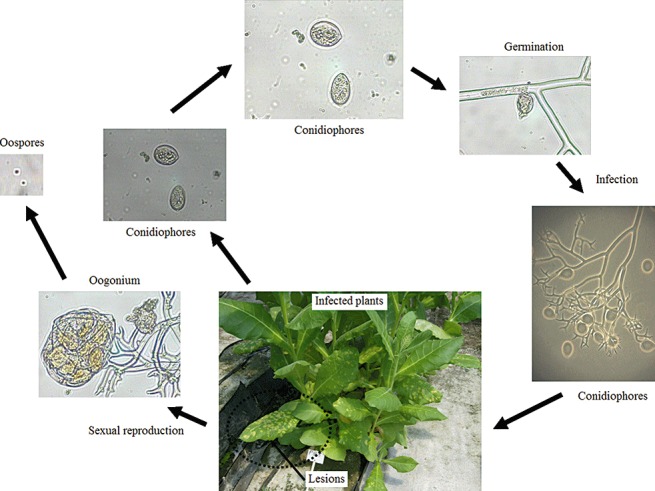
Disease cycle of tobacco blue mould caused by Peronospora hyoscyami f. sp. tabacina.
There are two basic ways in which the pathogen moves around. The first is by spores carried on the wind (Davis and Monahan, 1991; Lucas, 1980) and the second is by the movement, by people, of infected tobacco transplants (Aylor, 1986). Epidemics are usually cyclic and progressive; once established, they advance as a more or less definable front via wind‐borne spores. The difference between continuous and discontinuous epidemic fronts is related to inoculum dispersal patterns, localized weather, density and spatial aggregation of tobacco fields within a production region, and planting schedules. The pathogen is a prolific producer of spores; it has been estimated that 500 ha of heavily diseased tobacco can produce about 6.44 × 1013 spores per day (Aylor, 1986).
Once the air‐borne conidiophores land during the morning hours on a leaf surface, in the presence of free water germination and infection can occur in as little as 2–4 h. A 5‐ to 7‐day, symptom‐free incubation period takes place before the appearance of the first visible symptoms (yellow lesions) and sporulation. For conidiophores to appear, the relative humidity must exceed 95% for 3 h and darkness must last for a minimum of 1.5 h. Maximum sporulation occurs at 15–23 °C.
The oospores are sexual spores and have been suggested as another method of dissemination (Hall, 1989). However, it is unclear whether the pathogen is capable of overwintering in infected debris, and the role of oospores in disease is not clearly understood (Ristaino et al., 2007). They are sometimes produced in the mesophyll of dead parts of the infected plant (Lucas, 1975; Milholland et al., 1980). Mature oospores are usually reddish brown and 20–60 µm in diameter; their size varies under different conditions. Heist et al. (2002) observed that oospores of P. tabacina were produced on hyphae emerging from roots of N. repanda. This finding appears to be relevant to future studies of this pathosystem from the standpoint of potential secondary spread in commercial tobacco production systems and to address questions pertaining to the life cycle of P. tabacina in both wild and commercial Nicotiana species. The disease cycle of tobacco blue mould is shown in Fig. 2.
THE INFECTION PROCESS
The infection process of P. hyoscyami f. sp. tabacina typically begins with the germination of spores on the leaf surface, followed by the development of an appressorium (Lucas, 1975). The development of the appressorium depends on a thigmotrophic signal triggered by the specific topography of the host plant leaf surface (Lucas, 1975). An infection peg formed by the appressorium enters the leaf through a stomata, followed by the development of a substomatal vesicle, an infection hypha, a haustorial mother cell, penetration of a photosynthetic mesophyll cell by a peg and the establishment of a haustorium (Lucas, 1975). The development of the haustorium is the final step of an infection pathway in which the plant host plays a major role (Lucas, 1975).
The oomycete pathogen establishes intimate relations with its hosts by forming haustoria during the infection, which are well‐known structures that are thought to be used to obtain nutrients from the plant, redirecting host metabolism and suppressing host defences through the delivery of effector proteins into the host cytoplasm (Hahn and Mendgen, 2001; O'Connell and Panstruga, 2006; Voegele and Mendgen, 2003; Whisson et al., 2007). However, little is known about the biochemical and molecular events involved in this process during P. hyoscyami f. sp. tabacina–tobacco interaction. It would be interesting to determine the effectors mediating the compatible and incompatible interaction.
TOBACCO DEFENCE AGAINST BLUE MOULD INFECTION
Several defence responses have been reported to occur in the N. tabacum–P. hyoscyami f. sp. tabacina interaction (Alexander et al., 1993; Borrás‐Hidalgo et al., 2006; Kroumova et al., 2007; Lusso and Kuc, 1996; Salt et al., 1986; Schiltz, 1974). These include the activation of pathogenesis‐related (PR) genes, and an increase in the activities of typical PR proteins, such as peroxidases, chitinases, β‐1,3‐glucanases, lipoxygenases and T‐phylloplanin (Alexander et al., 1993; Kroumova et al., 2007; Lusso and Kuc, 1996). In addition, systemic acquired resistance is one of the best characterized tobacco defence responses activated on pathogen infection (McIntyre et al., 1981) (Table 1).
Table 1.
Tobacco genes involved in blue mould defence.
| Gene | Effect on disease development | Reference |
|---|---|---|
| β‐Ionone | Inhibits the sporulation and growth of P. hyoscyami f. sp. tabacina | Schiltz (1974) Salt et al. (1986) |
| PR‐1a | Overexpression in transgenic plants reduces rate and final disease | Alexander et al. (1993) |
| Glucanase | Overexpression in transgenic plants reduces disease symptoms | Lusso and Kuc (1996) |
| Glutathione synthetase and the EIL2 transcription factor | Knockdown of these genes in Nicotiana megalosiphon compromises disease resistance against P. hyoscyami f. sp. tabacina | Borrás‐Hidalgo et al. (2006) |
| T‐phylloplanin | Inhibits spore germination and disease | Kroumova et al. (2007) |
The sporulation and growth of P. hyoscyami f. sp. tabacina are inhibited by β‐ionone (Salt et al., 1986; Schiltz, 1974). β‐Ionone is a terpenoid volatile synthesized from carotenoids. It has been shown that T‐phylloplanin proteins secreted on the aerial surfaces of tobacco (N. tabacum) by short procumbent trichomes inhibit spore germination and blue mould disease caused by the oomycete pathogen P. hyoscyami f. sp. tabacina (Kroumova et al., 2007) (Table 1).
Tobacco plants with an N gene (resistance gene) inoculated on the lower leaves with tobacco mosaic virus (TMV) mount a systemic acquired resistance, which protects against P. hyoscyami f. sp. tabacina (McIntyre et al., 1981). Using a cultivar of tobacco (KY14) carrying the N gene, assays have shown that plants initially inoculated with TMV or with P. hyoscyami f. sp. tabacina exhibit increased activity of PR proteins, including β‐1,3‐glucanase (PR‐2), and this increased activity is associated with resistance to challenge inoculation with P. hyoscyami f. sp. tabacina (Ye et al., 1990). Evidence provided by transgenic plants suggests that PR‐2 has an important role in protecting plants against P. hyoscyami f. sp. tabacina (Ryals et al., 1996).
In addition, it has been reported that some genotypes of the desert tobacco N. obtusifolia respond to foliar P. hyoscyami f. sp. tabacina infections by developing necrotic lesions 5–6 days post‐inoculation, and that subsequent sporulation of the pathogen is drastically reduced or eliminated in this interaction compared with a compatible interaction. In addition, there is evidence that this resistance in N. obtusifolia results from the expression of the hypersensitive response (HR), and is caused by the action of a single, partially dominant gene named Rpt1 (Heist et al., 2004).
Finally, Nicotiana megalosiphon genes, which are involved in broad‐spectrum resistance to tobacco blue mould, have been identified in the incompatible interaction using suppression subtractive hybridization (Borrás‐Hidalgo et al., 2006). One hundred and eighty‐two clones were differentially expressed and 16% showed homology to known genes, some of which were implicated in disease resistance. Based on their expression profiles, some genes were chosen for functional analysis using virus‐induced gene silencing. Each was found to be quickly induced in the incompatible interaction on N. megalosiphon, whereas no induction was observed in the compatible interaction on N. tabacum. It was shown that a knockdown of the glutathione synthetase gene and the EIL2 transcription factor gene in N. megalosiphon compromised disease resistance against P. hyoscyami f. sp. tabacina (Borrás‐Hidalgo et al., 2006). This demonstrated that both components are required for P. hyoscyami defence, and could provide the basis for the disease‐resistant phenotype of N. megalosiphon.
DISEASE MANAGEMENT
Some important practices could be introduced in order to control tobacco blue mould. These include making the environment less favourable for the pathogen to survive and infect tobacco, keeping the pathogen out of tobacco and the area for as long as possible, protecting tobacco plants with fungicides when they are most vulnerable and managing the crop to harvest quickly.
The employment of host plant resistance is the most economic and environmentally sustainable method for controlling blue mould. However, naturally occurring resistance within N. tabacum is generally very low (Rufty, 1989). Resistance can be found within several Nicotiana species of Australian origin where blue mould is endemic, and the resistance has been introgressed into cultivated tobacco from N. debneyi (Clayton et al., 1967; Lea, 1963), N. goodspeedii Wheeler and N. velutina Wheeler (Wark, 1970). In Cuba, the non‐cultivated tobacco species N. megalosiphon has been shown to be highly resistant to P. hyoscyami f. sp. tabacina (Espino and Rey, 1987).
The incorporation of blue mould resistance into new cultivars has been complicated by the complex interaction between P. hyoscyami f. sp. tabacina and the tobacco host plant. Peronospora hyoscyami f. sp. tabacina is an obligate parasite, and selection for resistance is best performed under natural or induced field epidemics. Disease reactions are highly dependent, however, on factors such as plant age, physiological status of the plant and environmental conditions. These factors can cause field experiments to be highly variable and unpredictable (Rufty, 1989). Although DNA polymorphism, as revealed by molecular markers, is generally low for N. tabacum (Nishi et al., 2003), the identification of markers linked to resistance genes transferred to tobacco from wild Nicotiana relatives has been successful (Bai et al., 1995; Johnson et al., 2002; Lewis, 2005; Yi et al., 1998).
FUTURE PERSPECTIVES
In conclusion, the identification of molecular markers linked to genes contributing to blue mould resistance is a valuable tool to increase the capacity for the elimination of susceptible individuals and lines during early stages of breeding programmes. Moreover, knowledge about resistance genes to blue mould and the molecular characterization of P. hyoscyami f. sp. tabacina isolates will enhance our understanding of the resistance and pathogenicity process, respectively, allowing better strategies to be created to control blue mould disease.
REFERENCES
- Alexander, D. , Goodman, R.M. , Gut‐Rella, M. , Glascock, C. , Weymann, K. , Friedrich, L. , Madoox, D. , Ahl‐goy, P. , Luntz, T. , Ward, E. and Ryals, J. (1993) Increased tolerance to two oomycete pathogens in transgenic tobacco expressing pathogenesis‐related protein 1a. Plant Biol. 90, 7327–7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylor, D.E. (1986) A framework for examining inter‐regional aerial transport of fungal spores. Agric. Forest Meteorol. 38, 263–288. [Google Scholar]
- Bai, D. , Reeleder, R. and Brandle, J.E. (1995) Identification of two RAPD markers tightly linked with the Nicotiana debneyi gene for resistance to black root rot of tobacco. Theor. Appl. Genet. 91, 1184–1189. [DOI] [PubMed] [Google Scholar]
- Bashi, E. and Aylor, D.E. (1983) Survival of detached sporangia of Peronospora destructor and Peronospora tabacina . Phytopathology, 73, 1135–1139. [Google Scholar]
- Borrás‐Hidalgo, O. , Thomma, B.P.H.J. , Collazo, C. , Chacón, O. , Borroto, C.J. , Ayra, C. , Portieles, R. , López, Y. and Pujol, M. (2006) EIL2 transcription factor and glutathione synthetase are required for defense of tobacco against tobacco blue mold. Mol. Plant–Microbe Interact. 19, 399–406. [DOI] [PubMed] [Google Scholar]
- Clayton, E.E. , Heggestad, H.E. , Grosso, J.J. and Burk, L.G. (1967) The transfer of blue mold resistance to tobacco from Nicotiana debneyi . Toba. Sci. 11, 91–97. [Google Scholar]
- Cooke, M.C. (1891) Tobacco disease. Gard. Chron. 9, 173. [Google Scholar]
- Davis, J.M. and Monahan, J.F. (1991) Climatology of air parcel trajectories related to the atmospheric transport of Peronospora tabacina . Plant Dis. 75, 706–711. [Google Scholar]
- Dick, M.W. (2002) Towards an understanding of the evolution of the downy mildews In: Advances in Downy Mildew Research (Spencer‐Phillips P.T.N., Gisi U. and Lebeda A., eds.), pp. 1–57. Dordrecht: Kluwer. [Google Scholar]
- Espino, E. and Rey, X. (1987) Nuevas variedades de tabaco negro para cultivo bajo tela resistentes al moho azul (Peronospora tabacina). Agrotecnia, 19, 247–260. [Google Scholar]
- Hahn, M. and Mendgen, K. (2001) Signal and nutrient exchange at biotrophic plant fungus interfaces. Curr. Opin. Plant Biol. 4, 322–327. [DOI] [PubMed] [Google Scholar]
- Hall, G. (1989) Peronospora hyoscyami f. sp. tabacina . C.M.I. Descr. Pathog. Fungi Bact. 975, 1–3. [Google Scholar]
- Heist, E.P. , Nesmith, W.C. and Schardl, C.L. (2002) Interactions of Peronospora tabacina with roots of Nicotiana spp. in gnotobiotic associations. Phytopathology, 92, 400–405. [DOI] [PubMed] [Google Scholar]
- Heist, E.P. , Zaitlin, D. , Funnell, D.L. , Nesmith, W.C. and Schardl, C.L. (2004) Necrotic lesion resistance induced by Peronospora tabacina on leaves of Nicotiana obtusifolia . Phytopathology, 94, 1178–1188. [DOI] [PubMed] [Google Scholar]
- Johnson, E.S. , Wolff, M.S. and Wernsman, E.A. (2002) Marker‐assisted selection for resistance to black shank disease in tobacco. Plant Dis. 86, 1303–1309. [DOI] [PubMed] [Google Scholar]
- Julio, E. , Verrier, J.L. and Dorlhac de Borne, F. (2006) Development of SCAR markers linked to three disease resistances based on AFLP within Nicotiana tabacum L. Theor. Appl. Genet. 112, 335–346. [DOI] [PubMed] [Google Scholar]
- Kirk, P.M. , Cannon, P.F. , David, J.C. and Stalpers, J.A. (2001) Ainsworth & Bisby's Dictionary of the Fungi, 9th edn. Wallingford: CAB International. [Google Scholar]
- Kroumova, A.B. , Shepherd, R.W. and Wagner, G.J. (2007) Impacts of T‐phylloplanin gene knockdown and of Helianthus and Datura phylloplanins on Peronospora tabacina spore germination and disease potential. Plant Physiol. 144, 1843–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea, H.W. (1963) The transfer of resistance against blue mold (Peronospora tabacina Adam) from Nicotiana debneyi to cultivated tobacco. CORESTA Inf. Bull. 3, 13–15. [Google Scholar]
- Lewis, R.S. (2005) Transfer of resistance to potato virus Y (PVY) from Nicotiana africana to Nicotiana tabacum: possible influence of tissue culture on the rate of introgression. Theor. Appl. Genet. 110, 678–687. [DOI] [PubMed] [Google Scholar]
- Lucas, G.B. (1975) Diseases of Tobacco. Raleigh, NC: Biological Consulting Associates. [Google Scholar]
- Lucas, G.B. (1980) The war against blue mold. Science (Washington, DC) 210, 147–153. [DOI] [PubMed] [Google Scholar]
- Lusso, M. and Kuc, J. (1996) The effect of sense and antisense expression of the PR‐N gene for β‐1,3‐glucanase on disease resistance of tobacco to fungi and viruses. Physiol. Mol. Plant Pathol. 49, 267–283. [Google Scholar]
- Main, C.E. (1991) Blue mold In: Compendium of Tobacco Diseases (Shew H.D. and Lucas G.B. eds), pp. 5–9. St. Paul, MN: The American Phytopathological Society. [Google Scholar]
- McIntyre, J.L. , Dodds, J.A. and Hare, J.D. (1981) Effects of localized infections of Nicotiana tabacum by Tobacco Mosaic Virus on systemic resistance against diverse pathogens and an insect. Phytopathology, 71, 297–301. [Google Scholar]
- Milholland, R.D. , Papadopoulou, J. and Daykin, M. (1980) Histopathology of Peronospora tabacina in systemically infected burley tobacco. Phytopathology, 71, 73–76. [Google Scholar]
- Milla, S.R. , Levin, J.S. , Lewis, R.S. and Rufty, R.C. (2005) RAPD and SCAR markers linked to an introgressed gene conditioning resistance to Peronospora tabacina D.B. Adam. in tobacco. Crop Sci. 45, 2346–2354. [Google Scholar]
- Nesmith, W.C. (1984) The North American blue mold warning system. Plant Dis. 11, 933–936. [Google Scholar]
- Nishi, T. , Tajima, T. , Noguchi, S. , Ajisaka, H. and Negishi, H. (2003) Identification of DNA markers of tobacco linked to bacterial wilt resistance. Theor. Appl. Genet. 106, 765–770. [DOI] [PubMed] [Google Scholar]
- O'Connell, R.J. and Panstruga, R. (2006) Tete a tete inside a plant cell. Establishing compatibility between plants and biotrophic fungi and oomycetes. New Phytol. 171, 699–718. [DOI] [PubMed] [Google Scholar]
- Pérez, L. , Rodriguez, M.E. , Rodriguez, F. and Roson, C. (2003) Efficacy of acibenzolar‐S‐methyl, an inducer of systemic acquired resistance against tobacco blue mould caused by Peronospora hyoscyami f. sp. tabacina. Crop Prot. 22, 405–413. [Google Scholar]
- Reuveni, M. , Tuzun, S. , Cole, J.S. , Siegel, M.R. and Kuc, J. (1986) The effects of plant age and leaf position in the susceptibility of tobacco to blue mold caused by Peronospora tabacina . Phytopathology, 76, 455–458. [Google Scholar]
- Ristaino, J.B. , Johnson, A. , Blanco‐Meneses, M. and Liu, B. (2007) Identification of the tobacco blue mold pathogen, Peronospora tabacina, by polymerase chain reaction. Plant Dis. 91, 685–691. [DOI] [PubMed] [Google Scholar]
- Rotem, J. , Wooding, B. and Aylor, D.E. (1985) The role of solar radiation, especially ultraviolet, in the mortality of fungal sores. Phytopathology, 75, 510–514. [Google Scholar]
- Rufty, R.C. (1989) Genetics of host resistance to tobacco blue mold In: Blue Mold of Tobacco (McKeen W.E. ed.). pp. 141–164. St. Paul, MN: The American Phytopathological Society. [Google Scholar]
- Ryals, J.A. , Neuenschwander, U.H. , Willits, M.G. , Molina, A. , Steiner, H.Y. and Hunt, M.D. (1996) Systemic acquired resistance. Plant Cell, 8, 1809–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt, S.D. , Tuzun, S. and Kuc, J. (1986) Effect of β‐ionone and abscisic acid on the growth of tobacco and resistance to blue mold: mimicry of effects of stem infection by Peronospora tabacina Adam. Physiol. Mol. Plant Pathol. 28, 287–297. [Google Scholar]
- Schiltz, P. (1974) Action inhibitrice de la β‐ionone au cours du développement de Peronospora tabacina . Ann. Tabac. 11, 207–216. [Google Scholar]
- Schiltz, P. (1981) Downy mildew of tobacco In: The Downy Mildews (Spencer D.M., ed.), pp. 577–599. London: Academic Press. [Google Scholar]
- Shepherd, C. (1970) Nomenclature of the tobacco blue mold fungus. Trans. Br. Mycol. Soc. 55, 253–256. [Google Scholar]
- Voegele, R.T. and Mendgen, K. (2003) Rust haustoria: nutrient uptake and beyond. New Phytol. 159, 93–100. [DOI] [PubMed] [Google Scholar]
- Voglmayr, H. (2008) Progress and challenges in systematics of downy mildews and white blister rusts: new insights from genes and morphology. Eur. J. Plant Pathol. 122, 3–18. [Google Scholar]
- Wark, D.C. (1970) Development of flue‐cured tobacco cultivars resistant to a common strain of blue mold. Toba. Sci. 14, 147–150. [Google Scholar]
- Whisson, S.C. , Boevink, P.C. , Moleleki, L. , Avrova, A.O. , Morales, J.G. , Gilroy, E.M. , Armstrong, M.R. , Grouffaud, S. , Van West, P. , Chapman, S. , Hein, I. , Toth, I.K. , Pritchard, L. and Birch, P.R.J. (2007) A translocation signal for delivery of oomycete effector proteins into host plant cells. Nature, 450, 115–118. [DOI] [PubMed] [Google Scholar]
- Wolf, F.A. , Dixon, L.F. , McLean, R. and Darkis, F.R. (1934) Downy mildew of tobacco. Phytopathology, 24, 337–363. [Google Scholar]
- Ye, X.S. , Pan, S.Q. and Kuc, J. (1990) Association of pathogenesis‐related proteins and activities of peroxidase, β‐1,3‐glucanase and chitinase with systemic induced resistance to blue mould of tobacco but not to systemic tobacco mosiac virus. Physiol. Mol. Plant Pathol. 36, 523–531. [Google Scholar]
- Yi, Y.H. , Rufty, R.C. and Wernsman, E.A. (1998) Identification of RAPD markers linked to the wildfire resistance gene of tobacco using bulked segregant analysis. Toba. Sci. 42, 52–57. [Google Scholar]


