Figure 4.
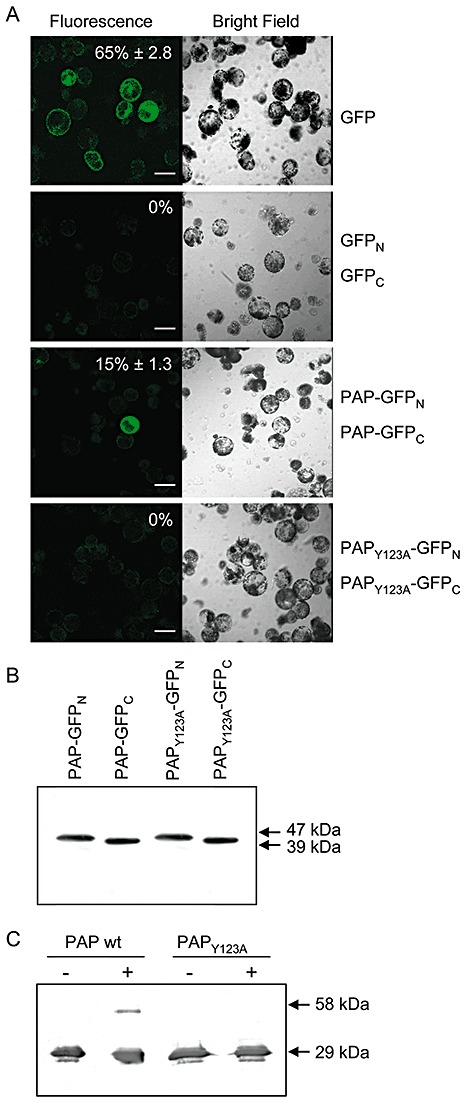
Bimolecular fluorescence complementation of pokeweed antiviral protein (PAP) dimerization in pokeweed protoplasts. Protoplasts were transfected with plasmids encoding the N‐ or C‐terminus of green fluorescent protein (GFP) fused to PAP or PAPY123A. As a negative control for bimolecular interaction, cells were transfected with plasmids encoding only the N‐ or C‐terminus of GFP. As a positive control for fluorescence, protoplasts were transfected with a plasmid encoding intact GFP. Transfected protoplasts were examined by fluorescence or bright field laser scanning microscopy. Percentage values indicate the mean numbers of fluorescent protoplasts relative to the total number of cells ± standard error from five fields of view. The scale bar indicates 50 µm. (B) Expression of individual constructs, PAP–GFPN, PAP–GFPC, PAPY123A–GFPN and PAPY123A–GFPC, following transfection into pokeweed protoplasts. Lysates of protoplasts (10 µg) were separated by 12% sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE), transferred to nitrocellulose and probed with a polyclonal antibody specific to GFP (1:5000). The molecular weights of fusion proteins containing the N‐terminus of GFP (47 kDa) and the C‐terminus of GFP (39 kDa) are indicated. (C) In vitro chemical cross‐linking. Recombinant wild‐type PAP and PAPY123A (125 ng) were incubated with or without (+, −) 0.05% glutaraldehyde and separated by 12% SDS‐PAGE with prior boiling. Proteins were transferred to nitrocellulose and probed with a polyclonal antibody specific to PAP (1:5000). Molecular weights of monomeric (29 kDa) and complexed (58 kDa) PAP are indicated.
