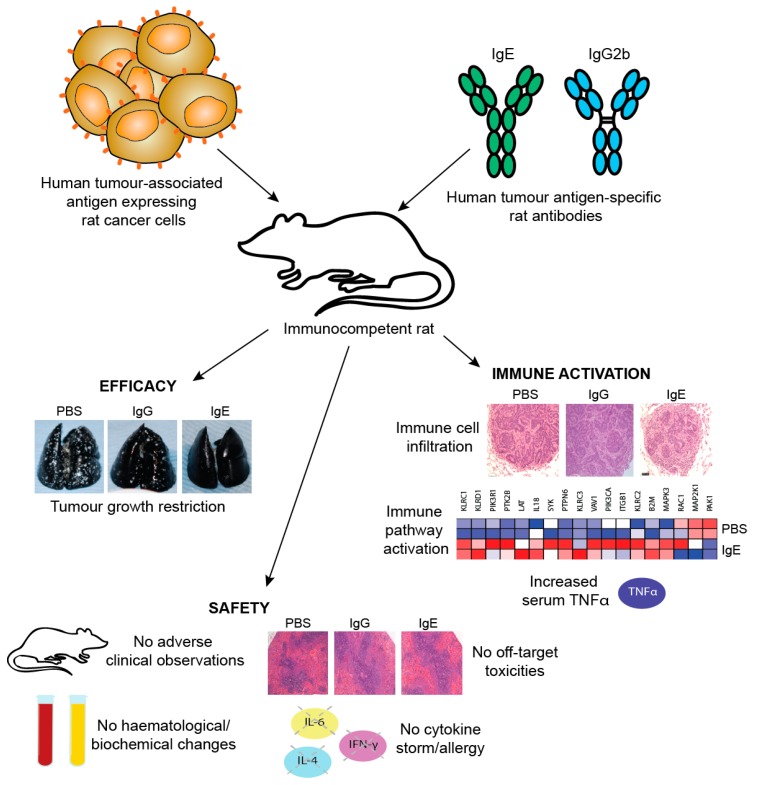
Figure 13.
In vivo safety evaluations of MOv18 IgE. A surrogate syngeneic tumour model in immunocompetent WAG rats was designed to evaluate the safety profile of MOv18 IgE. Rat CC531 colon adenocarcinoma cells, engineered to express the human FRα, were administered i.v. to grow as lung metastases and animals were treated with either rat MOv18 IgE or the IgG2b equivalent. This model demonstrated the superior efficacy of IgE compared with the IgG counterpart (representative images of Indian ink-stained lungs shown). Efficacy was observed in the absence of any adverse clinical observations, off-target toxicities (H&E-stained spleen shown), or haematological or biochemical changes. Furthermore, no evidence of cytokine storm (lack of IL-6 or IFNγ upregulation) or allergic response (lack of IL-4 upregulation) was detected. In the same model, MOv18 IgE treatment was associated with the restriction of tumour growth, alongside enhanced immune cell infiltration in tumours (H&E-stained lung shown) and elevated immunological pathway activation gene signatures. Additionally, increased tumour and serum TNFα were measured in association with IgE treatment. Figure adapted by permission from John Wiley & Sons, Inc. (Josephs, D.H. et al. An immunologically relevant rodent model demonstrates safety of therapy using a tumour-specific IgE. Allergy 2018, 73, 2328–2341 [191]).