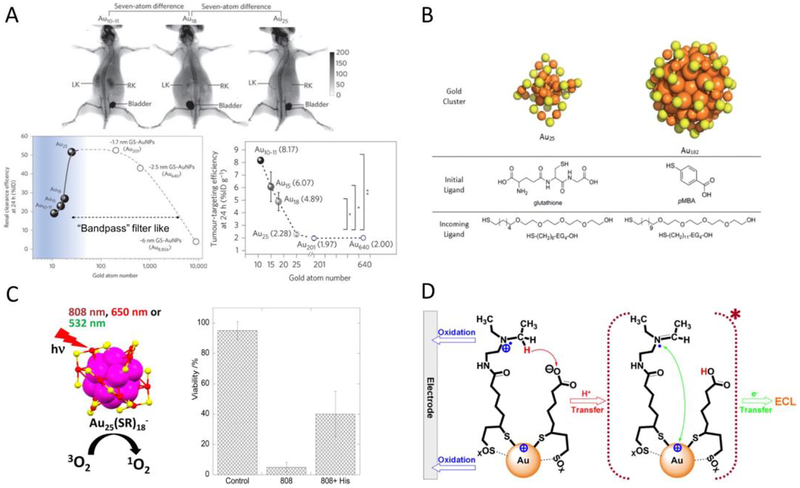
Fig.2.
(A) Whole-body X-ray images of mice after i.v. injection of Au10–11, Au18 and Au25 at 40min post injection. The smallest Au10–11 is retained in kidney much longer than Au18, which is in turn longer than Au25, although all three AuNCs are cleared through kidneys into the bladder (top). Renal clearance efficiency of glutathione protected Au10–11, Au15, Au18, Au25, 1.7nm (~Au201), 2.5nm (~Au640) and 6nm (~Au8856) AuNPs at 24h post i.v. injection, embodying the kidney glomerulus filtration as an atomically precise ‘bandpass” barrier (bottom left). Passive tumor targeting efficiency of glutathione protected Au10–11, Au15, Au18, Au25, Au201 and Au640 at 24h post i.v. injection (bottom right). (B) Structures of each molecular component used in the ligand replacement study. (C) Schematic illustration of efficient singlet oxygen generation by Au25(SR)18 nanoclusters under visible/NIR (532, 650, and 808nm) light irradiation (left). In vitro experiments demonstrate Au25(Capt)18 - nanoclusters can efficiently kill Hela cells photodynamically under 808nm light irradiation (right). (D) Scheme of stepwise NIR ECL generation from lipoic acid stabilized Au22 nanoclusters (Au-LA) conjugated with coreactants N,N-diethylethylenediamine (DEDA). (Adapted or reprinted with permissions from Springer Nature for (A) Ref. [63], Royal Society of Chemistry for (B) Ref. [64], American Chemical Society for (C) Ref. [66] and (D) Ref. [85].)