Peripheral artery disease (PAD) is a progressive form of atherosclerosis that affects the lower extremities, particularly in patients with diabetes. Critical limb ischemia (CLI), the end-stage manifestation of this disease, is associated with an increased risk of chronic leg ulcerations, infections, amputations, and mortality. At presentation, 30% of CLI patients require amputation, and 25% will die within 1 year (1). Toxic metal exposure, particularly to cadmium, has been associated with an increased risk of vascular disease in epidemiological studies and PAD severity in patients with coronary artery disease (CAD) (2–4). Toxic metals are ubiquitous and can induce increased oxidative stress, endothelial dysfunction, and inflammation (4). Similarly, chronic cadmium exposure has been found to play a role in the acceleration of vascular disease in animal models (5,6). Edetate disodium is a chelating agent with high affinity for lead and cadmium (7). The Trial to Assess Chelation Therapy (TACT) demonstrated that edetate disodium–based chelation reduced cardiovascular events, especially in patients with diabetes after myocardial infarction (8,9).
Case Presentation
An 81-year-old man with a 35-year history of insulin-dependent diabetes, a 20 pack-year smoking history (quit date October 2001), atrial fibrillation, and CAD requiring coronary artery bypass in 2001 presented to the vascular service. His medications at baseline included sotalol, rivaroxaban, pentoxifylline, clopidogrel, aspirin, insulin, pregabalin, and atorvastatin. His baseline serum creatinine was 0.77 mg/dL, and his BMI was 22 kg/m2.
He had progressive CLI with nonhealing ulcers and pain unimproved by medical therapy. Noninvasive tests demonstrated abnormal ankle-brachial indices bilaterally. Lower-extremity angiography revealed total occlusions of the left external iliac artery, left superficial femoral artery, distal right superficial femoral artery, and right popliteal artery.
Vascular Surgery performed a left femoral thrombo-endarterectomy and patch angioplasty (August 2015), followed by a left ilio-femoral endarterectomy with vein patch repair of the left common femoral artery 3 months before the top row of images in Figure 1 were taken (December 2016). The ulcers progressively worsened despite hyperbaric oxygen therapy (Figure 1, top row). Physical examination revealed atrophic and cool lower extremities bilaterally, with absent pedal pulses bilaterally. His WIfI (wound, ischemia, and foot infection risk stratification) score was 320, with a high risk for amputation and high revascularization benefit.
FIGURE 1.
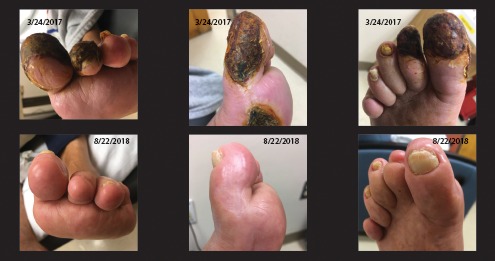
Evolution of wound appearance in a patient at baseline (top) and infusion 48 (bottom). Last revascularization procedure was 3 months before top row of images were taken, resulting in progressive deterioration.
Dry gangrene had developed in the first and second phalanges extending to the medial aspect of the foot (Figure 1, top row). The patient was reevaluated by Vascular Surgery and Interventional Radiology. Both services concluded that they could offer no additional endovascular or surgical options. Given his continued ischemic rest pain, the patient was offered a below-the-knee amputation for palliation.
We offered the patient a series of 50 intravenous infusions of up to 3 g of edetate disodium–based chelation as prepared in TACT (10). The peripheral artery study was approved by the institutional review board and carried out under the U.S. Food and Drug Administration investigational new drug exemption for edetate disodium. Conventional therapy, including wound care and medications, continued throughout the study. Infusions were given twice weekly for the first 10 weeks, followed by 20 weekly infusions. The final 10 infusions were administered monthly, for a total of 50 infusions. Oral multivitamins and multiminerals were given daily throughout the duration of the study, as used in TACT (10). Urine was collected in metal-free containers before and after the first infusion. Urine toxic metals, normalized to urine creatinine, were measured by inductively coupled plasma mass spectrometry (Doctors Data, Inc., St. Charles, Ill.). The PAD Questionnaire and the 36-item Short Form Health Survey (SF-36) were completed to assess disease-specific quality of life during the treatment (11,12). The patient’s A1C was 7.5% at baseline and 7.0% after 6 months.
Urine measurements demonstrated a 2,773% increase in urine lead and a 428% increase in urine cadmium over baseline, normalized as microgram of metal per gram of urine creatinine. All toxic metals analyzed are found in Table 1. After 40 infusions, the patient’s quality of life had improved compared to baseline (SF-36 Physical Function by 70 points, General Health by 30 points; PAD Questionnaire Summary Scale by 50 points). The pain component of the SF-36 improved by 91 points, with no pain at the end of the study. Gangrene and foot ulcers resolved (Figure 1, bottom row). During treatment, the patient did not have any major cardiovascular events (myocardial infarction, amputations, revascularizations, or death) or infections requiring antibiotic therapy.
TABLE 1.
Baseline Toxic Metals
| Baseline | After Edetate Disodium Infusion | Percentage Change | |
|---|---|---|---|
| Toxic metals in urine, µg metal/g creatinine* | |||
| Aluminum | 2.4 | 22 | 816 |
| Antimony | 0.3 | 0.5 | 67 |
| Arsenic | 33 | 17 | –48 |
| Barium | 3.3 | 2.7 | –18 |
| Beryllium | <dl | <dl | — |
| Bismuth | <dl | <dl | — |
| Cadmium | 0.7 | 3.7 | 428 |
| Cesium | 12 | 9.1 | –24 |
| Gadolinium | 0.2 | 32 | 15,900 |
| Lead | 0.6 | 17 | 2,733 |
| Mercury | 0.7 | 1 | 43 |
| Nickel | 9.4 | 16 | 70 |
| Palladium | <dl | <dl | — |
| Platinum | <dl | <dl | — |
| Tellurium | <dl | <dl | — |
| Thallium | 0.2 | 0.2 | 0 |
| Thorium | <dl | <dl | — |
| Tin | 36 | 520 | 1,344 |
| Tungsten | 0.6 | 0.2 | –66 |
| Uranium | <dl | <dl | — |
| sUrine creatinine, mg/dL | 59.7 | 103 | 72 |
All measured by inductively coupled plasma mass spectrometry and collected in metal-free containers. <dl, below the method detection limit.
Questions
Can patients with diabetes and CLI who have exhausted standard medical, catheter-based, and surgical interventions benefit from edetate disodium–based chelation infusion therapy?
If edetate disodium–based treatment does improve severe PAD in patients with diabetes, is maintenance treatment necessary to maintain the benefit?
What are the potential mechanisms of action of the benefit observed and how was blood flow restored in this case?
Commentary
Patients with diabetes and PAD all too often develop CLI and undergo subsequent amputation, preceded by multiple surgical and endovascular procedures. We report on a patient with diabetes and CLI who had undergone unsuccessful surgical attempts at revascularization. A regimen targeted to chelate vasculotoxic metals improved his symptoms and quality of life, restored tissue, and resulted in ulcer resolution.
Toxic metals such as cadmium and lead, by definition, have no role in the human body. As an example, cadmium is a toxic atherogenic metal that enters the human body through smoking, contaminated food, and water. Similarly, lead is also vasculotoxic, causing endothelial dysfunction and replacing calcium in some intracellular reactions. Both of these metals are stored in the body with a long half-life of approximately 30 years.
Epidemiological studies support the association of toxic metals and PAD (2,4). An environmental association study from the National Health and Nutrition Examination Survey identified cadmium as being independently associated with PAD (13). Chronic cadmium exposure accelerates atherosclerosis and increases total cholesterol in animal models (6). Our group recently reported that PAD severity is associated with increased urine cadmium levels in patients with CAD (3).
Edetate disodium is a chelating agent with a high affinity for cadmium and lead and may reduce body stores of these metals, potentially decreasing their vasculotoxic effects (7). Although appealing as an explanation for the present case report, the toxic metal chelation hypothesis is not yet established science. For example, we cannot exclude the potential impact of calcium-lowering by edetate disodium and possible arterial decalcification. By the same token, if this hypothesis is correct, other chelators might show even greater activity in CLI.
The National Institutes of Health–funded TACT showed a nearly 50% reduction in recurrent myocardial infarction and a 43% reduction in mortality in the subgroup of patients with diabetes (8). In conjunction with the present case, these findings suggesting improvement in vascular health should stimulate further research into an otherwise intractable disease. Ultimately, a larger, well-powered, placebo-controlled trial will be needed to define or discard edetate disodium as potential salvage therapy before amputation in patients with diabetes and CLI.
Clinical Pearls
Patients with diabetes and CLI and no endovascular or surgical options may be candidates for edetate disodium treatment.
The TACT proved that edetate disodium–based chelation is safe when administered according to the TACT protocol.
Although there has been an association between toxic metals (iron, cadmium, and arsenic) and cardiovascular disease, it is unknown whether targeted therapy to decrease body stores of these metals results in benefits. A large clinical trial is needed to help answer this question.
Acknowledgments
Duality of Interest
No potential conflicts of interest relevant to this article were reported.
Author Contributions
F.U. and I.A.A. researched the data and wrote the manuscript. T.Y., R.B., and D.D., reviewed and edited the manuscript. G.A.L. conceived the study, secured funding, and reviewed and edited the manuscript. G.A.L. is the guarantor of this work and, as such, had full access to all data reported and takes responsibility for the accuracy of the content.
References
- 1.Iida O, Takahara M, Soga Y, Azuma N, Nanto S, Uematsu M; Investigators PRIORITY. Prognostic impact of revascularization in poor-risk patients with critical limb ischemia: the PRIORITY registry (Poor-Risk Patients With and Without Revascularization Therapy for Critical Limb Ischemia). JACC Cardiovasc Interv 2017;10:1147–1157 [DOI] [PubMed] [Google Scholar]
- 2.Tellez-Plaza M, Guallar E, Fabsitz RR, et al. . Cadmium exposure and incident peripheral arterial disease. Circ Cardiovasc Qual Outcomes 2013;6:626–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ujueta F, Arenas IA, Diaz D, et al. . Cadmium level and severity of peripheral artery disease in patients with coronary artery disease. Eur J Prev Cardiol 2018. Epub ahead of print (doi: 10.1177/2047487318796585) [DOI] [PubMed] [Google Scholar]
- 4.Navas-Acien A, Selvin E, Sharrett AR, Calderon-Aranda E, Silbergeld E, Guallar E. Lead, cadmium, smoking and increased risk of peripheral arterial disease. Circulation 2004;109:3196–3201 [DOI] [PubMed] [Google Scholar]
- 5.Messner B, Knoflach M, Seubert A, et al. . Cadmium is a novel and independent risk factor for early atherosclerosis mechanisms and in vivo relevance. Arterioscler Thromb Vasc Biol 2009;29:1392–1398 [DOI] [PubMed] [Google Scholar]
- 6.Oliviera TF, Batista PR, Leal MA, et al. . Chronic cadmium exposure accelerates the development of atherosclerosis and induces vascular dysfunction in the aorta of ApoE-/- mice. Biol Trace Element Res. 29 April 2018. Epub ahead of print (doi: 10.1007/s12011-018-1359-1) [DOI] [PubMed]
- 7.Arenas IA, Navas-Acien A, Ergui I, Lamas GA. Enhanced vasculotoxic metal excretion in post-myocardial infarction patients following a single edetate disodium-based infusion. Envion Res 2017;158:443–449 [DOI] [PubMed] [Google Scholar]
- 8.Escolar E, Lamas GA, Mark DB, et al. . The effect of an EDTA-based chelation regimen on patients with diabetes mellitus and prior myocardial infarction in the Trial to Assess Chelation Therapy (TACT). Circulation 2014;7:15–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamas GA, Goertz C, Boineau R, et al. . Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: the TACT randomized trial. JAMA 2013;309:1241–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamas GA, Goertz C, Boineau R, et al. . Design of the Trial to Access Chelation Therapy. Am Heart J 2012;163:7–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spertus J, Jones P, Poler S, Rocha-Singh K. The Peripheral Artery Questionnaire: a new disease-specific health status measure for patients with peripheral arterial disease. Am Heart J 2004;147:301–308 [DOI] [PubMed] [Google Scholar]
- 12.Ware JE, Sherbourne D. The MOS 36-item short-form health survey (SF-36). Med Care 1992;30:473–483 [PubMed] [Google Scholar]
- 13.Zhuang X, Ni A, Liao L, et al. . Environment-wide association study to identify novel factors associated with peripheral arterial disease: evidence from the National Health and Nutrition Examination Survey (1999–2004). Atherosclerosis 2018;269:172–177 [DOI] [PubMed] [Google Scholar]


