Abstract
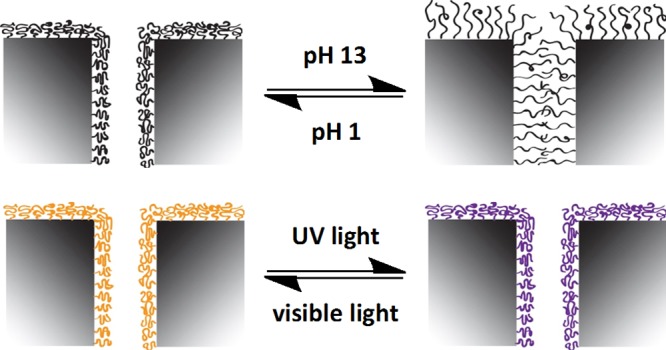
A microporous pH- and light-responsive membrane that enables remote control over its interfacial properties has been fabricated. pH-Responsiveness was imparted to a porous polypropylene film via grafting of poly(methacrylic acid) brushes from the substrate using argon-plasma-induced free-radical graft polymerization. Morphological changes as a function of grafting level were analyzed using atomic force microscopy. Conversion into a light-responsive membrane was performed via postpolymerization modification to covalently attach photochromic spiropyran moieties to the grafted polymer brushes. Reversible switches in wettability and permeability were determined upon changing from acidic to basic pH or upon alternating UV- and visible-light irradiation. Additionally, light-responsive membranes show a switch in color upon UV exposure.
Introduction
Smart membranes that are sensitive to their environment have received tremendous attention in recent years as they enable rapid, remote-controlled switching of their interfacial properties.1−7 They are materials designed with properties such as swelling behavior, permeability, or interactions with ions and biomolecules that can be changed reversibly in a controlled way by external stimulation. Switching originates from responsiveness to a certain trigger such as changes in pH,8 temperature,9 concentration of metal ions,10 or exposure to light.11
Polypropylene (PP) membranes are low-cost materials that show advanced bulk properties in terms of their mechanical strength, dimensional stability, and chemical inertness,12 which brings with them, however, a degree of hydrophobicity, low reactivity, and poor biocompatibility. Modification of such commercial substrates to improve their surface properties is, therefore, very attractive.13 Conventional simple methods include coating and grafting processes. The readily implemented dip- or spin-coating techniques have the disadvantages of pore blockage and weak adhesion forces to the parent polymer.14 Grafting of polymer brushes covalently either via “grafting-to” or via “grafting-from” is a very powerful, efficient, and simple method to covalently attach new functionalities allowing a fast response to an environmental stimulus due to flexible mobile chain ends, while retaining the desirable bulk properties of the parent material. Different techniques have been established as a pretreatment to activate polymeric membranes for graft polymerization, namely, via plasma,15 ozone,16 UV light,17 or γ-irradiation.18 Plasma activation is a fast, dry, and environmentally friendly approach, which has been heavily exploited in the industry for the cleaning of surfaces and recently also for surface activation to form homogenous coatings.19,20
The adjustment of pH conditions is a simple and low-energy method,21 which has resulted in its becoming the most commonly used stimulus in membrane science. Membranes that are sensitive toward pH have been fabricated using a variety of processes including the formation of polymer networks, chemical modification of the bulk material, or via grafting of polymer brushes on their surfaces. Typical pH-responsive polymers are weak polyelectrolytes, that is, polymerized weak acids such as poly(acrylic acid)21 and poly(methacrylic acid) (PMAA)22 or bases such as poly(N,N-dimethylaminoethylmethacrylate)23 or poly(4-vinylpyridine).24 They undergo conformational transitions depending on the pH conditions. The response of polyacids to pH occurs via deprotonation above their particular pKa values, which induces a swelling of the charged polymer and—when grafted to a porous membrane—a decrease in the pore size. Alternatively, protonation below their pKa values induces a collapse of the noncharged polymer and an increase in pore size.25 pH-Responsive membranes have been used for chemical separation processes such as for oil/water mixtures,26 proteins,27 and metal ions28 as well as in biotechnological applications such as biosensors,4 drug-delivery systems,29 or nonfouling surfaces.30
However, research regarding light-responsive polymeric membranes has been exceedingly rare.2 As a stimulus, light is very attractive because it can be applied as an external trigger,31 allowing remote-controlled actuation of closed systems without risk of chemical contamination. Photons can be easily focused and delivered over long distances to a well-defined surface area. Photochromic materials undergo light-induced conformational changes, usually inducing a change in dipole moment. The best investigated photochromic moieties used as reversible organic switches in polymeric materials are azobenzenes32 and spiropyrans (SPs).33,34 SP performs a heterolytic ring cleavage under UV-light irradiation to form the planar zwitterionic merocyanine (MC) isomer, which produces—when attached to a surface—a dramatic change in surface free energy, resulting in enhanced wettability. The MCs close back to the nonpolar SP form upon exposure to visible light or thermally induced relaxation. Light-responsive materials have found applications in photonic devices and35 photosensitive lenses36 and have been used to control permeability,11,37 protein adhesion,38 and drug delivery.39−41
There are in general two strategies to graft light-responsive polymer brushes onto porous polymeric membranes. One approach is via a random graft-copolymerization of photochromic monomers, and another approach is using postpolymerization modification (PPM) of previously grafted polymer brushes.42 PPM benefits from its simplicity, as commercially available monomers can be used without the need to synthesize photochromic monomers or to optimize polymerization conditions for such highly functional monomers.43−45
In this article, we demonstrate a facile approach to grafting functional polymer brushes on microporous polymeric substrates. PMAA polymer brushes were grafted from a 30 μm-thick PP membrane to endow it with pH-responsiveness. Further, an established PPM45 of the grafted polymer brushes with photochromic SPs was used to create light-responsive polymer brushes (PMA–SP). Changes in wettability and in permeability upon change in pH and visible- and UV-light irradiation were analyzed using static water contact angle (CA) and water-flux measurements.
This study shows the powerful potential for facile modification methods such as plasma-induced growth of polymer brushes on inert polymer membranes. Using highly efficient PPM methods, a broad range of properties such as nonbiofouling, catalytic property, or bioactivity can be applied by conjugating, for example, enzymes or antibodies.
Results and Discussion
Grafting of pH-Responsive PMAA Polymer Brushes on Polymeric Substrates
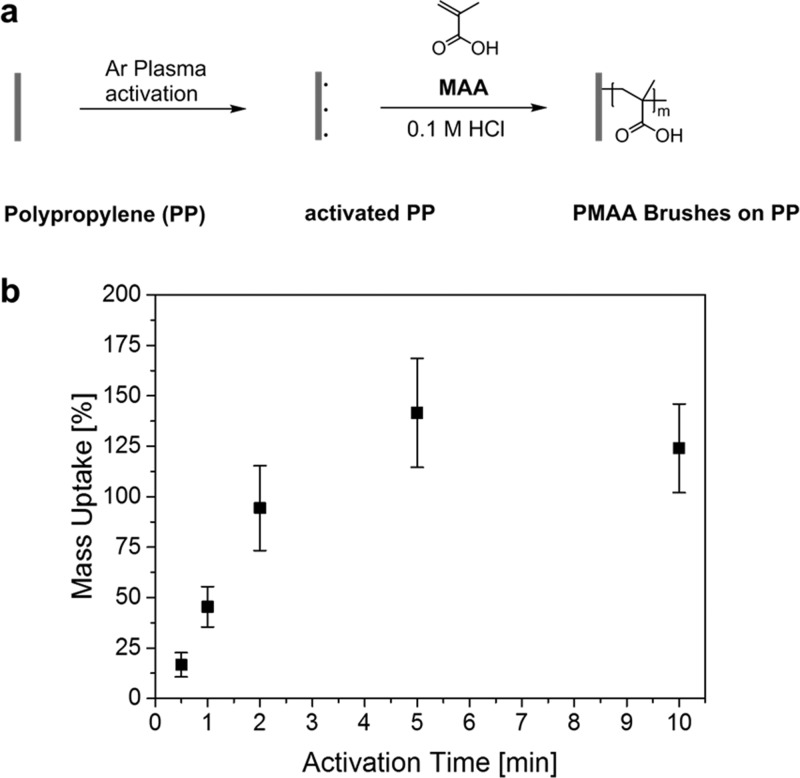
In this work, we used an argon plasma source to asymmetrically activate a microporous PP membrane and, for comparison, a flat PP film. The asymmetric activation leads to a gradient of grafted structures through the porous membrane. Figure 1a illustrates the activation step and the subsequent grafting of pH-responsive PMAA polymer brushes from the polymeric substrates via free-radical polymerization (FRP). In Figure 1b, the grafting level (GL)—that is, the mass uptake of PP membrane samples after the grafting of PMAA polymer brushes—is shown as a function of the argon-plasma activation time. The GL achieved under the same reaction conditions was found to increase with increasing activation time, as a consequence of an increasing radical concentration. It reached a maximum after 4 min and decreased again with longer activation time. This indicates that a very high radical density enhances side reactions in the grafting process, such as the recombination of surface-bound or chain-end radicals in the immediate vicinity, leading to the termination of the polymerization process. Similar behavior was observed by Shi et al.22 when grafting PMAA polymer brushes on polyethersulfone (PES) substrates.
Figure 1.
(a) Strategy for grafting of PMAA polymer brushes after one-side argon plasma activation from PP substrates and (b) mass uptake of PP membranes after grafting of PMAA polymer brushes as a function of activation time.
Atomic force microscopy (AFM) height images of a nongrafted membrane sample and samples grafted under identical conditions with 30 s, 1 min, and 2 min activation times are shown in Figure 2. With the applied FRP technique, polymer grafting led to a drastic change in morphology. With increasing GL, the fibers of the pristine microporous PP membrane changed from a very delicate microporous network to an increasingly closed system (see also Figure S3). Furthermore, samples of higher GL showed optical inhomogeneity and became brittle and opaque. The static water CAs of grafted PMAA on both PP membrane and flat-pressed PP film samples have been determined with respect to activation time with argon plasma (Figure S1). After grafting with hydrophilic PMAA, the CA was drastically reduced from 117° to 36° for membrane samples and from 103° to 57° for films, independent of their activation time with argon plasma. The difference in CA between the membrane and film samples was caused by their different morphologies, the higher surface roughness of the membrane samples leading to Cassie–Baxter wetting.
Figure 2.
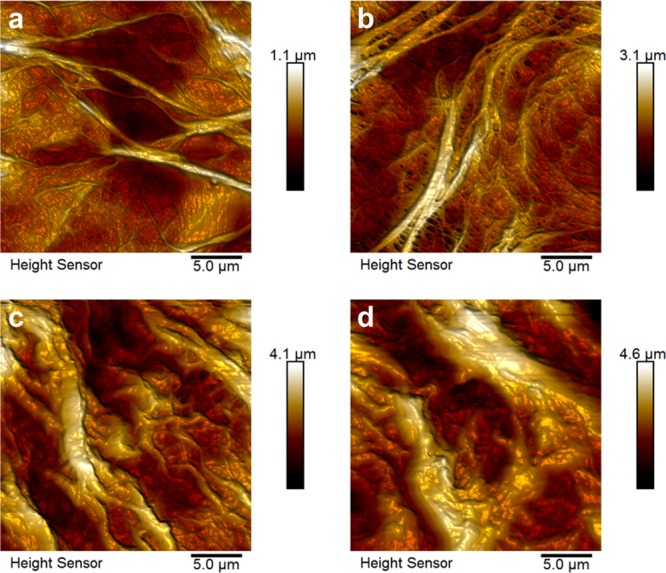
AFM images of (a) a pristine, nongrafted PP membrane and PMAA polymer brushes grafted from a PP membrane after (b) 30 s, (c) 1 min, and (d) 2 min activation time with argon plasma.
Switching Properties of pH-Responsive PMAA Polymer Brushes
The response of PMAA polymer brushes in terms of wettability at different pH values was determined qualitatively by static water CA measurements. Figure 3a shows that the CA could be reversibly switched between 59° and <10° for PP membranes and between 68° and 21° for PP films, upon alternating acidic and basic conditions. These changes in wettability are caused by a change in the surface free energy due to reversible protonation from the more hydrophobic state (PMAA) under acidic conditions and deprotonation of the carboxylic acids to the very hydrophilic polyelectrolyte state (PMA–) under basic conditions. Under neutral conditions, the CA in both cases took up a position in between both states (36° for membranes and 58° for film samples), which could be interpreted as a configuration where the brushes are partly deprotonated to a partially deprotonated polyelectrolyte P(MAA/MA–) (Figure 3b). However, to calculate the switch in the surface free energy of such hydrophilic surfaces, dynamic CA measurements need to be carried out, taking potential reorientations of the flexible surface-bound chains into account.46 The pH-responsiveness in terms of permeability of PMAA-modified PP membranes has been determined by measuring the flux under neutral, acidic, and basic conditions and as a function of the GL caused by different activation times with argon plasma (Table 1, and Figure 4).
Figure 3.
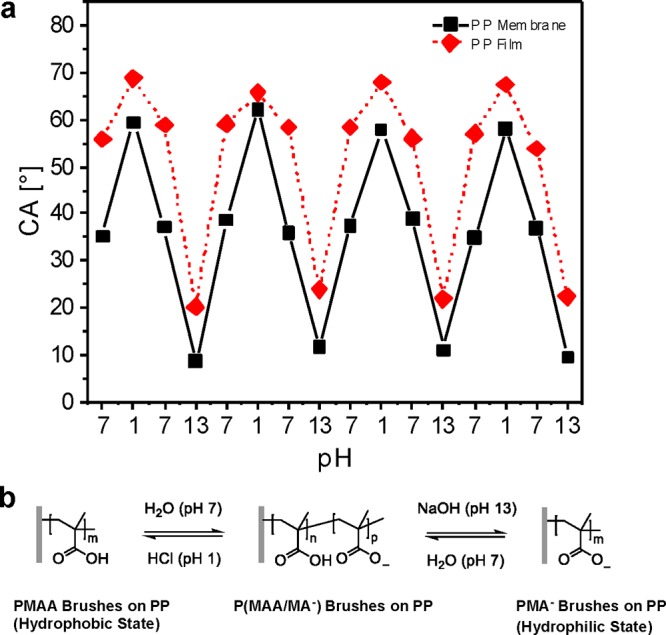
Reversible pH-induced switching (a) static water CAs of PMMA-brush-modified PP membrane and film surfaces measured with 3 μL water droplets between (b) the fully protonated hydrophobic state (PMAA) under acidic conditions, a partially deprotonated polyelectrolyte state (P(MAA/MA–)) under neutral pH, and the fully deprotonated polyelectrolyte configuration (PMA–) under basic conditions. The error in the values is estimated to be below 2°.
Table 1. Flux with Respect to the GL Caused by the Activation Time with Argon Plasma of (a) PMAA-Brush-Modified PP Membranes under Different pH Conditions and of (b) PMA–SP Brush-Modified PP Membranes after Visible- or UV-Light Exposure.
| (activation time [min]) | 0 | 0.5 | 1 | 2 | 5 |
|---|---|---|---|---|---|
| grafting level [%] | 0 | 17 ± 6 | 46 ± 10 | 93 ± 21 | 142 ± 27 |
| pH 13, flux [L m–2 h–1] | 308 ± 6 | 94 ± 8 | 68 ± 8 | 47 ± 8 | 55 ± 9 |
| pH 1, flux [L m–2 h–1] | 308 ± 5 | 331 ± 4 | 183 ± 7 | 117 ± 6 | 100 ± 7 |
| pH switch [%] | − | 252 | 168 | 148 | 81 |
| visible light, flux [L m–2 h–1] | 308 ± 5 | 289 ± 8 | 253 ± 7 | 240 ± 6 | 236 ± 6 |
| UV light, flux [L m–2 h–1] | 306 ± 5 | 405 ± 4 | 348 ± 5 | 319 ± 5 | 315 ± 5 |
| light switch [%] | − | 40 | 37 | 33 | 34 |
Figure 4.
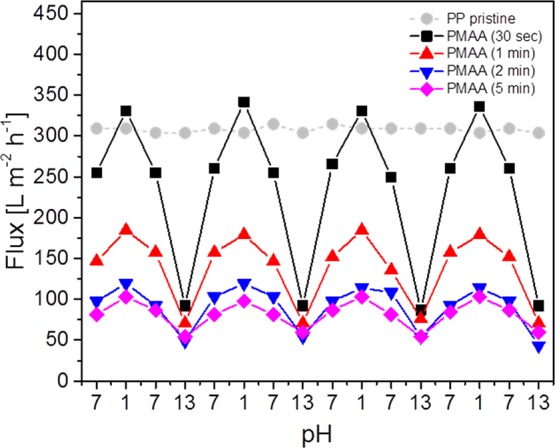
Reversible switching of the flux through PMMA-brush-modified PP membranes under different pH conditions with respect to the GL, which in turn is a function of the activation time with argon plasma. The most intense switching is observed at a lowest GL. The error in the values is estimated to be below 3 L m–2 h–1.
The water flux of a PMAA-grafted PP membrane (d = 12 mm) with a 30 s plasma activation (256 L m–2 h–1) was lower than that of the pristine PP membrane (306 L m–2 h–1), indicating a reduced pore size caused by the grafting of PMAA brushes. Under acidic conditions, the flux is enhanced by roughly 30% compared with the neutral pH, to 331 L m–2 h–1, that is, to a value higher than that of the pristine membrane. This effect is interpreted as deswelling of the fully protonated PMAA brushes, combined with the higher wettability of PMAA compared with PP (CAPP = 117° vs CAPMAA = 36°). Under basic conditions, the flux was strongly reduced by 63% compared with that in pH 7, to −94 L m–2 h–1, caused by the full deprotonation of the carboxylic acid moieties, causing a strong swelling of the polymer brushes (Figure 4). This switching under alternating pH conditions was reversible for more than 10 cycles. With increasing GL, the water flux under neutral conditions reduced significantly, that is, by more than two-thirds, from 256 L m–2 h–1 for a 30 s activation time to 81 L m–2 h–1 for 5 min activation time. Similar trends in the flux upon swelling and deswelling of the polymer brushes under basic and acidic conditions were observed for all GLs. The relative flux at low pH was roughly 10–20% higher than that under aqueous conditions, independent of the GL. This implies a similar deswelling of the polymer brushes, irrespective of their GL. This could be a consequence of a number of side reactions, leading to a higher polydispersity and a potential crosslinking of grafted polymer chains according to higher radical densities on the surface. By contrast, the relative flux under basic conditions is more dependent on the GL compared with that under aqueous conditions. It reduced from 63% for 30 s to 32% for 5 min activation time because the membrane pores became increasingly blocked. Furthermore, at longer exposure times, side reactions such as termination through recombination of surface-bound radicals and crosslinking could lead to an increasing inflexibility and lower swelling rates of the grafted networks. In summary, the highest magnitude of flux switching due to pH was determined at the lowest GL (252% for 30 s activation) and the lowest magnitude was determined at the highest GL (81% for 5 min activation).
Postpolymerization of PMAA Polymer Brushes To Create Light-Responsive Substrates
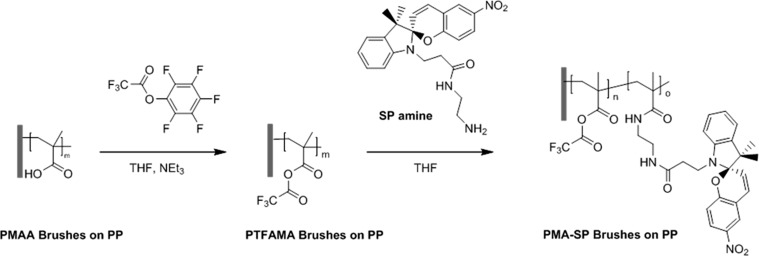
To impart light responsiveness to the PP substrates, a previously established two-step PPM was applied to covalently attach photochromic SP moieties via an amine linker to the activated PMAA polymer brushes (Figure 5).
Figure 5.
Strategy for the synthesis of light-responsive polymer brushes via PPM of PMAA polymer structures on PP substrates.
In this process, the carboxylic acid groups of the PMAA brushes are first converted in quantitative yields into fluorinated anhydrides [poly(trifluoroacetic methacrylate anhydride) (PTFAMA)].45 The activated carboxylic groups allow covalent attachment of the SP amines via the formation of an amide to modify the polymer brushes with up to 40% yield. Detailed characterization of the grafted PMAA polymer brushes and their PPM on both PP membranes and films using attenuated total reflectance infrared (ATR-IR) microscopy and CA measurements are given in Figure S2 and Tables S1 and S2.
Switching Properties of Light-Responsive Polymeric Substrates
After the PPM, the PP membrane samples appeared light pink and showed a color switch to deep purple when exposed to UV light, inducing the SP–MC transition (Figure 6a). The color change was also detected in UV/vis transmission spectrometry as an absorbance band at 570 nm (Figure 6b). Upon alternating exposure to visible and UV light, the static water CA of the SP-modified surfaces could be reversibly switched between 88° (PMMA–SP) and 66° (PMMA–MC) on PP films and between 103° and 93° on PP membranes (Figure 7a). The surface free energy increases because of the increased dipole moment of the MC form, causing a higher wettability and a lower CA. The switches in CA on both surfaces are within the range reported for SP-modified surfaces.44,45 Again, the difference in CA between the membrane and film samples is presumably an effect of their differing morphologies.
Figure 6.
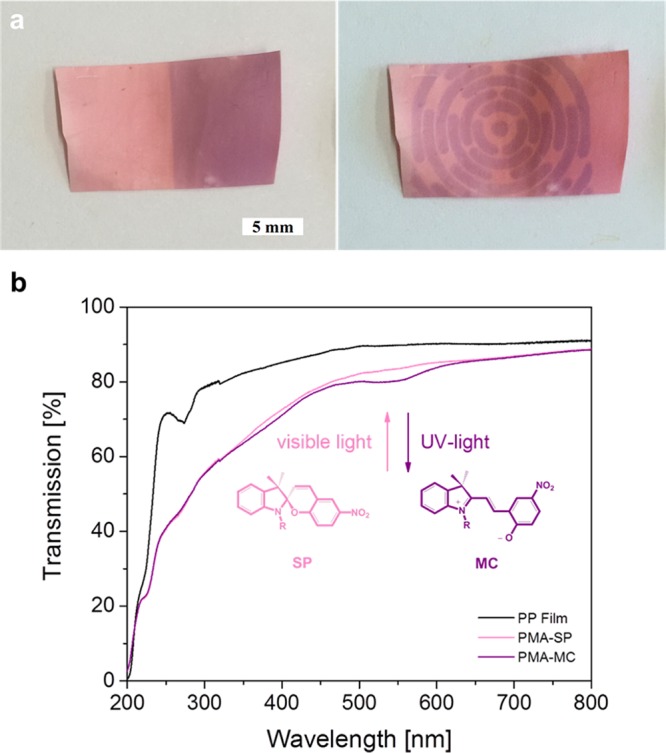
(a) Optical images of consecutive light-induced color switches on a photochromic PP membrane according to the transformation of isomeric structures of ring-closed SP to the zwitterionic open form of MC. In areas covered by different masks, light pink PMA–SP brushes do not switch in color, whereas in areas exposed to UV light, the membrane appears deep purple because of PMA–MC brushes. (b) Transmission spectra of thick PMA–SP brushes on a PP film, upon exposure to visible (PMA–SP) and UV (PMA–MC) light, showing the reversible switch in absorption around 570 nm.
Figure 7.
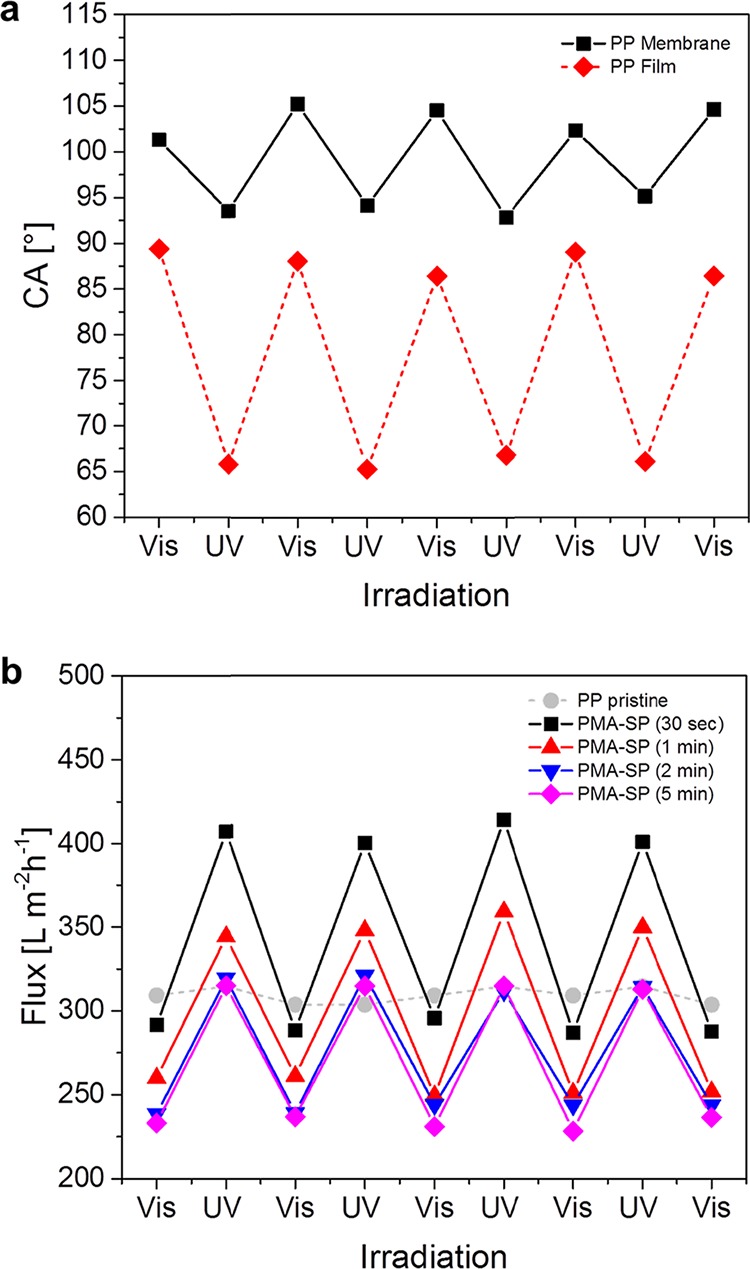
Representative reversible switching in (a) static water CA on a PMA–SP-brush-modified PP membrane and film, measured with 3 μL water droplets and in (b) water flux through a pristine and an SP-modified PP membrane under a pressure of 750 mbar upon alternating exposure to visible and UV light. The error in the values is estimated to be below 2° for CA and below 3 L m–2 h–1 for flux measurements.
In Figure 7b and Table 1, the switching of permeability upon light exposure of photochromic PP membranes is demonstrated using flux measurements under alternating UV- and visible-light irradiation. The flux of a photochromic membrane with the lowest GL increased by 40% from 289 to 405 L m–2 h–1 after an exposure of 30 s with UV light and relaxed back within 30 min under ambient visible light. A similar, but much weaker, UV-light-induced permeability increase has been described by Chung et al.11 for grafted SP-containing methacrylates on poly(tetrafluoroethylene) (PTFE), involving a reversible switch of 15%.
With increasing GL, the average flux was reduced under both visible- and UV-light irradiation, corresponding to the reduced pore sizes. In contrast to the pH-dependent switch in flux, the permeability of UV-sensitive membranes appears not to be dominated by the swelling behavior of the brushes but by the wettability switch induced by the SP–MC transition. The usually short activation time of only 30 s with UV light causes an immediate difference in wetting but not in swelling. The swelling of the zwitterionic brushes would need more time, as the chains have to decoil, a process associated with configurational changes. The pristine and SP-modified PP membranes are very hydrophobic, whereas the zwitterionic MC isomers formed under UV-light irradiation favor the passage of water through the membrane. This hypothesis is supported by the rather minor changes in the relative switch in permeability for light-responsive membranes, which reduced only from 40 to 33% with increasing GL, whereas the change for pH-responsive membranes was from 252 to 81%.
Methods
Materials
Commercial 30 μm-thick microporous PP membranes (TreoPore-PDA 30, Treofan Germany GmbH & Co. KG, Raunheim, Germany) with a porosity of >60% and hot-pressed, flat 120 μm-thick PP films (Kolma 59464, Kolma AG, Wabern, Switzerland) were used as substrates and were rinsed with ethanol before use. Methacrylic acid (Sigma-Aldrich, Buchs, Switzerland) was purified by distillation. Other chemicals were purchased from Sigma-Aldrich (Buchs, Switzerland), VWR (Zurich, Switzerland), or Fluka (Buchs, Switzerland) and were used as received. Water for rinsing or as a solvent was of Millipore quality (Quantum Ex/Q-Gard 2, Merck Millipore, Zug, Switzerland). The SP amine was prepared as previously reported.45
Sample Preparation
PP substrates were activated with low-pressure argon plasma (Femto, Diener Electronics, Jettingen, Germany) operated at 40 kHz/30 W for between 30 s and 5 min, similarly to previously described procedures.44,45 Plasma-treated samples were allowed to stand in air for 10 min before grafting and placed in a monomer solution of methacrylic acid (5 vol %) in 6 mL of 0.1 M HCl, degassed with nitrogen, and heated for 1 h at 70 °C. For the postpolymerization, PMAA brush samples were reacted in a solution of 196 μL of triethylamine (1.4 mmol) and 244 μL of perfluorophenyl trifluoroacetate (1.4 mmol) in 6 mL of tetrahydrofuran (THF) for 24 h at room temperature (RT) and subsequently in a solution of 85 mg of SP amine (0.2 mmol) in 6 mL of THF for 24 h at RT.
Atomic Force Microscopy
AFM scans in height mode were acquired via Tapping Mode in air on a Dimension Icon instrument (Bruker, Karlsruhe, Germany) using proprietary silicon nitride (Si3N4) cantilevers with a tip radius of 7 nm, a spring constant of 26 N m–1, and a resonance frequency of 300 kHz. Images were processed with second-order flattening procedures (Nanoscope software, Veeco, Germany).
Attenuated Total Reflectance Infrared Spectroscopy
Measurements were recorded using a Hyperion 3000 IR microscope (Bruker, Fällanden, Switzerland) equipped with an ATR objective with a circular contact area of 100 μm diameter.
Water Contact Angle Determination
A DataPhysics OCA 25 contact angle measuring system (DataPhysics GmbH, Filderstadt, Germany) was used for the determination of static CAs of 3 μL water droplets.
Flux Measurements
Membrane samples were placed between two PE frits (20 μm, Carl Roth GmbH+ Co. KG, Karlsruhe, Germany) in a 6 mL SPE column (PP, Carl Roth GmbH+ Co. KG, Karlsruhe, Germany). The column end was connected to a vacuum pump (SC920, KNF Lab, KNF Neuberger GmbH, Freiburg, Germany) to produce a minor under-pressure of 750 mbar. Samples were placed in the relevant aqueous solutions for 1 h before measurements. For pH-responsive measurements, the flux was determined by the time taken for 5 mL of water, HCl (0.1 M), or NaOH (0.1 M) to pass through the membrane. The method used generally leads to higher relative errors when determining high flow rates. For light-responsive measurements, samples were exposed for 30 s to UV light before use.
UV/Vis Spectroscopy
A UV/vis/near-infrared spectrometer (Lambda19, PerkinElmer, Schwerzenbach, Switzerland), equipped with a deuterium lamp and a halogen light source, was used for acquiring the UV/vis transmission spectra in the range of 200 to 800 nm.
Conclusions
A new method for the fabrication of responsive polymeric membranes has been demonstrated that uses argon-plasma activation and FRP to graft pH-responsive PMAA polymer brushes from microporous PP. It was clearly evident from the ATR-IR spectra that the PMAA polymer brushes had been grafted and functionalized via a two-step PPM strategy to generate photochromic SP-containing PMA–SP polymer brushes. Both PMAA- and PMA–SP-modified membranes were successfully shown to be smart materials that could switch in wettability and permeability in response to either pH or light as external stimulus. Switching was demonstrated using static water CA and water-flux measurements with respect to their GL. In addition, photochromic PMA–SP brushes show a reversible switch in color upon alternating visible- and UV-light irradiation. The flux properties of pH-switched PMAA-modified membranes were dominated by the swelling of the brushes and influenced little by the hydrophilicity of the surface. By contrast, hydrophilicity changes dominated the photon-induced switching of the SP-modified membranes.
The advantage of the presented approach lies in its simplicity and versatility. The application of responsive systems to influence hydrophilicity or pore size via swelling of the material is of great interest for the controlled separation or transport of dissolved species.
Acknowledgments
The authors thank Camelia Borca for assistance with IR microscopy, Yves Erdin for assistance with sample preparation, and Lorenz Gubler for providing the membrane substrates. The authors gratefully acknowledge the financial support from the Swiss National Science Foundation (SNF).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.6b00394.
Static water CA measurements of PMAA-brush-grafted PP membranes and films, ATR-IR spectra and CA measurements of the PPM of PMAA polymer brushes grafted onto PP membranes and films, respectively (PDF)
Author Present Address
§ Department of Chemical Engineering, Aristotle University of Thessaloniki, University Campus, 54124 Thessaloniki, Greece (M.-E.N).
The authors declare no competing financial interest.
Supplementary Material
References
- Cabane E.; Zhang X.; Langowska K.; Palivan C. G.; Meier W. Stimuli-responsive polymers and their applications in nanomedicine. Biointerphases 2012, 7, 9. 10.1007/s13758-011-0009-3. [DOI] [PubMed] [Google Scholar]
- Nicoletta F. P.; Cupelli D.; Formoso P.; De Filpo G.; Colella V.; Gugliuzza A. Light responsive polymer membranes: A review. Membranes 2012, 2, 134–197. 10.3390/membranes2010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C.; Nie S.; Tang M.; Sun S. Polymeric pH-sensitive membranes—A review. Prog. Polym. Sci. 2011, 36, 1499–1520. 10.1016/j.progpolymsci.2011.05.004. [DOI] [Google Scholar]
- Stuart M. A. C.; Huck W. T. S.; Genzer J.; Müller M.; Ober C.; Stamm M.; Sukhorukov G. B.; Szleifer I.; Tsukruk V. V.; Urban M.; Winnik F.; Zauscher S.; Luzinov I.; Minko S. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. 10.1038/nmat2614. [DOI] [PubMed] [Google Scholar]
- Tokarev I.; Minko S. Stimuli-responsive porous hydrogels at interfaces for molecular filtration, separation, controlled release, and gating in capsules and membranes. Adv. Mater. 2010, 22, 3446–3462. 10.1002/adma.201000165. [DOI] [PubMed] [Google Scholar]
- Wandera D.; Wickramasinghe S. R.; Husson S. M. Stimuli-responsive membranes. J. Membr. Sci. 2010, 357, 6–35. 10.1016/j.memsci.2010.03.046. [DOI] [Google Scholar]
- Dai S.; Ravi P.; Tam K. C. Thermo- and photo-responsive polymeric systems. Soft Matter 2009, 5, 2513–2533. 10.1039/b820044k. [DOI] [Google Scholar]
- Zhang Q.; Xia F.; Sun T.; Song W.; Zhao T.; Liu M.; Jiang L. Wettability switching between high hydrophilicity at low pH and high hydrophobicity at high pH on surface based on pH-responsive polymer. Chem. Commun. 2008, 1199–1201. 10.1039/b716681h. [DOI] [PubMed] [Google Scholar]
- Reber N.; Kuchel A.; Spohr R.; Wolf A.; Yoshida M. Transport properties of thermo-responsive ion track membranes. J. Membr. Sci. 2001, 193, 49–58. 10.1016/s0376-7388(01)00460-4. [DOI] [Google Scholar]
- Liu Z.; Luo F.; Ju X.-J.; Xie R.; Luo T.; Sun Y.-M.; Chu L.-Y. Positively K+-Responsive Membranes with Functional Gates Driven by Host–Guest Molecular Recognition. Adv. Funct. Mater. 2012, 22, 4742–4750. 10.1002/adfm.201201251. [DOI] [Google Scholar]
- Chung D.-J.; Ito Y.; Imanishi Y. Preparation of porous membranes grafted with poly(spiropyran-containing methacrylate) and photocontrol of permeability. J. Appl. Polym. Sci. 1994, 51, 2027–2033. 10.1002/app.1994.070511207. [DOI] [Google Scholar]
- Yang Q.; Xu Z.-K.; Dai Z.-W.; Wang J.-L.; Ulbricht M. Surface modification of polypropylene microporous membranes with a novel glycopolymer. Chem. Mater. 2005, 17, 3050–3058. 10.1021/cm048012x. [DOI] [Google Scholar]
- Nebhani L.; Barner-Kowollik C. Orthogonal transformations on solid substrates: Efficient avenues to surface modification. Adv. Mater. 2009, 21, 3442–3468. 10.1002/adma.200900238. [DOI] [Google Scholar]
- Wang N.; Zhang G.; Ji S.; Qin Z.; Liu Z. The salt-, pH- and oxidant-responsive pervaporation behaviors of weak polyelectrolyte multilayer membranes. J. Membr. Sci. 2010, 354, 14–22. 10.1016/j.memsci.2010.03.002. [DOI] [Google Scholar]
- Yu W. H.; Kang E. T.; Neoh K. G. Controlled grafting of comb copolymer brushes on poly(tetrafluoroethylene) films by surface-initiated living radical polymerizations. Langmuir 2005, 21, 450–456. 10.1021/la0485531. [DOI] [PubMed] [Google Scholar]
- Ying L.; Yu W. H.; Kang E. T.; Neoh K. G. Functional and surface-active membranes from poly(vinylidene fluoride)-graft-poly(acrylic acid) prepared via RAFT-mediated graft copolymerization. Langmuir 2004, 20, 6032–6040. 10.1021/la049383v. [DOI] [PubMed] [Google Scholar]
- Yu H.-Y.; Li W.; Zhou J.; Gu J.-S.; Huang L.; Tang Z.-Q.; Wei X.-W. Thermo- and pH-responsive polypropylene microporous membrane prepared by the photoinduced RAFT-mediated graft copolymerization. J. Membr. Sci. 2009, 343, 82–89. 10.1016/j.memsci.2009.07.012. [DOI] [Google Scholar]
- Deng B.; Yang X.; Xie L.; Li J.; Hou Z.; Yao S.; Liang G.; Sheng K.; Huang Q. Microfiltration membranes with pH dependent property prepared from poly(methacrylic acid) grafted polyethersulfone powder. J. Membr. Sci. 2009, 330, 363–368. 10.1016/j.memsci.2009.01.010. [DOI] [Google Scholar]
- Barbey R.; Lavanant L.; Paripovic D.; Schüwer N.; Sugnaux C.; Tugulu S.; Klok H.-A. Polymer brushes via surface-initiated controlled radical polymerization: Synthesis, characterization, properties, and applications. Chem. Rev. 2009, 109, 5437–5527. 10.1021/cr900045a. [DOI] [PubMed] [Google Scholar]
- Hegemann D.; Brunner H.; Oehr C. Plasma treatment of polymers for surface and adhesion improvement. Nucl. Instrum. Methods Phys. Res., Sect. B 2003, 208, 281–286. 10.1016/S0168-583X(03)00644-X. [DOI] [Google Scholar]
- Sinha M. K.; Purkait M. K. Preparation and characterization of novel pegylated hydrophilic pH responsive polysulfone ultrafiltration membrane. J. Membr. Sci. 2014, 464, 20–32. 10.1016/j.memsci.2014.03.067. [DOI] [Google Scholar]
- Shi Q.; Su Y.; Ning X.; Chen W.; Peng J.; Jiang Z. Graft polymerization of methacrylic acid onto polyethersulfone for potential pH-responsive membrane materials. J. Membr. Sci. 2010, 347, 62–68. 10.1016/j.memsci.2009.10.006. [DOI] [Google Scholar]
- Schacher F.; Ulbricht M.; Müller A. H. E. Self-supporting, double stimuli-responsive porous membranes from polystyrene-block-poly(N,N-dimethylaminoethyl methacrylate) diblock copolymers. Adv. Funct. Mater. 2009, 19, 1040–1045. 10.1002/adfm.200801457. [DOI] [PubMed] [Google Scholar]
- Qiu X.; Yu H.; Karunakaran M.; Pradeep N.; Nunes S. P.; Peinemann K.-V. Selective separation of similarly sized proteins with tunable nanoporous block copolymer membranes. ACS Nano 2013, 7, 768–776. 10.1021/nn305073e. [DOI] [PubMed] [Google Scholar]
- Chu L.; Xie R.; Ju X. Stimuli-responsive membranes: Smart tools for controllable mass-transfer and separation processes. Chin. J. Chem. Eng. 2011, 19, 891–903. 10.1016/S1004-9541(11)60070-0. [DOI] [Google Scholar]
- Xiang Y.; Shen J.; Wang Y.; Liu F.; Xue L. A pH-responsive PVDF membrane with superwetting properties for the separation of oil and water. RSC Adv. 2015, 5, 23530–23539. 10.1039/c5ra00739a. [DOI] [Google Scholar]
- Chakrabarty T.; Shahi V. K. Modified chitosan-based, pH-responsive membrane for protein separation. RSC Adv. 2014, 4, 53245–53252. 10.1039/c4ra05314a. [DOI] [Google Scholar]
- Hasegawa S.; Ohashi H.; Maekawa Y.; Katakai R.; Yoshida M. Thermo- and pH-responsive poly(A-ProOMe)-graft-poly(AAc) membrane for selective separation of metal ions. Polym. J. 2009, 41, 533–540. 10.1295/polymj.PJ2007219. [DOI] [Google Scholar]
- Järvinen K.; Åkerman S.; Svarfvar B.; Tarvainen T.; Viinikka P.; Paronen P. Drug release from pH and ionic strength responsive poly(acrylic acid) grafted poly(vinylidenefluoride) membrane bags in vitro. Pharm. Res. 1998, 15, 802–805. 10.1023/A:1011995725320. [DOI] [PubMed] [Google Scholar]
- Tripathi B. P.; Dubey N. C.; Choudhury S.; Simon F.; Stamm M. Antifouling and antibiofouling pH responsive block copolymer based membranes by selective surface modification. J. Mater. Chem. B 2013, 1, 3397–3409. 10.1039/c3tb20386g. [DOI] [PubMed] [Google Scholar]
- Klajn R. Spiropyran-based dynamic materials. Chem. Soc. Rev. 2014, 43, 148–184. 10.1039/C3CS60181A. [DOI] [PubMed] [Google Scholar]
- Shinkai S.; Kinda H.; Manabe O. Photoresponsive complexation of metal cations with an azobenzene-crown-azobenzene bridge immobilized in polymer supports. J. Am. Chem. Soc. 1982, 104, 2933–2934. 10.1021/ja00374a045. [DOI] [Google Scholar]
- Berkovic G.; Krongauz V.; Weiss V. Spiropyrans and spirooxazines for memories and switches. Chem. Rev. 2000, 100, 1741–1754. 10.1021/cr9800715. [DOI] [PubMed] [Google Scholar]
- Minkin V. I. Photo-, thermo-, solvato-, and electrochromic spiroheterocyclic compounds. Chem. Rev. 2004, 104, 2751–2776. 10.1021/cr020088u. [DOI] [PubMed] [Google Scholar]
- Irie M. Photochromism: Memories and Switches—Introduction. Chem. Rev. 2000, 100, 1683–1684. 10.1021/cr980068l. [DOI] [PubMed] [Google Scholar]
- Bouas-Laurent H.; Dürr H. Organic Photochromism (IUPAC Technical Report). Pure Appl. Chem. 2001, 73, 639–665. 10.1351/pac200173040639. [DOI] [Google Scholar]
- Sumaru K.; Ohi K.; Takagi T.; Kanamori T.; Shinbo T. Photoresponsive properties of poly(N-isopropylacrylamide) hydrogel partly modified with spirobenzopyran. Langmuir 2006, 22, 4353–4356. 10.1021/la052899+. [DOI] [PubMed] [Google Scholar]
- Nayak A.; Liu H.; Belfort G. An optically reversible switching membrane surface. Angew. Chem., Int. Ed. 2006, 45, 4094–4098. 10.1002/anie.200600581. [DOI] [PubMed] [Google Scholar]
- Gong C.; Wong K.-L.; Lam M. H. W. Photoresponsive molecularly imprinted hydrogels for the photoregulated release and uptake of pharmaceuticals in the aqueous media. Chem. Mater. 2008, 20, 1353–1358. 10.1021/cm7019526. [DOI] [Google Scholar]
- Baumann L.; de Courten D.; Wolf M.; Rossi R. M.; Scherer L. J. Light-responsive caffeine transfer through porous polycarbonate. ACS Appl. Mater. Interfaces 2013, 5, 5894–5897. 10.1021/am401218e. [DOI] [PubMed] [Google Scholar]
- Baumann L.; Schöller K.; de Courten D.; Marti D.; Frenz M.; Wolf M.; Rossi R. M.; Scherer L. J. Development of light-responsive porous polycarbonate membranes for controlled caffeine delivery. RSC Adv. 2013, 3, 23317–23326. 10.1039/c3ra44399j. [DOI] [Google Scholar]
- Burns C. T.; Choi S. Y.; Dietz M. L.; Firestone M. A. Acidichromic spiropyran-functionalized mesoporous silica: Towards stimuli-responsive metal ion separations media. Sep. Sci. Technol. 2008, 43, 2503–2519. 10.1080/01496390802122311. [DOI] [Google Scholar]
- Gauthier M. A.; Gibson M. I.; Klok H.-A. Synthesis of functional polymers by post-polymerization modification. Angew. Chem., Int. Ed. 2008, 48, 48–58. 10.1002/anie.200801951. [DOI] [PubMed] [Google Scholar]
- Dübner M.; Gevrek T. N.; Sanyal A.; Spencer N. D.; Padeste C. Fabrication of thiol–ene “clickable” copolymer-brush nanostructures on polymeric substrates via extreme ultraviolet interference lithography. ACS Appl. Mater. Interfaces 2015, 7, 11337–11345. 10.1021/acsami.5b01804. [DOI] [PubMed] [Google Scholar]
- Dübner M.; Spencer N. D.; Padeste C. Light-responsive polymer surfaces via post-polymerization modification of grafted polymer-brush structures. Langmuir 2014, 30, 14971–14981. 10.1021/la503388j. [DOI] [PubMed] [Google Scholar]
- Grundke K.; Bogumil T.; Werner C.; Janke A.; Pöschel K.; Jacobasch H.-J. Liquid–fluid contact angle measurements on hydrophilic cellulosic materials. Colloids Surf., A 1996, 116, 79–91. 10.1016/0927-7757(96)03587-x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.