Abstract
Background:
Highly porous surfaces promoting biologic fixation have renewed interest in cementless total knee arthroplasty (TKA), but the potential for failed biologic fixation remains. The purpose of this study was to compare the clinical outcomes of cemented and cementless versions of the same TKA design at an average of 2 years postoperatively.
Methods:
This was an institutional review board-approved, prospective, randomized controlled trial of patients from 18 to 75 years of age who were undergoing a primary TKA. Patients with inflammatory arthritis, a body mass index (BMI) of >40 kg/m2, infection, a neuromuscular disorder, or grossly osteoporotic bone or bone defects were excluded. Patients were randomized to receive a cemented or cementless cruciate-retaining TKA of the same design. The cementless implant has highly porous fixation surfaces. Oxford Knee, Knee Society, and Forgotten Joint Scores were collected. Patients were asked to rate the knee with the TKA as a percentage of normal. Power analysis indicated that 130 patients were necessary to demonstrate a 5-point difference in the Oxford Knee Score at 90% power.
Results:
One hundred and forty-seven patients were enrolled, and 141 (96%) of them were analyzed at an average of 2 years postoperatively. There was no difference in age, sex, BMI, American Society of Anesthesiologists (ASA) score, or duration of follow-up (p = 0.1 to 0.9). There was also no difference in the change in the hemoglobin level from the preoperative measurement to postoperative day 1 between the 2 cohorts (mean and standard deviation, −2.6 ± 1.4 g/dL compared with −2.5 ± 0.9 g/dL, p = 0.5), but the total operative time was decreased in the cementless cohort (82.1 ± 16.6 compared with 93.7 ± 16.7 minutes, p = 0.001). There were no differences in any clinical outcome measure at 4 to 6 weeks, 1 year, or an average of 2 years postoperatively (p = 0.1 to 0.9) between the cemented and cementless cohorts. There was no radiographic evidence of component subsidence or loosening in either cohort.
Conclusions:
This study demonstrated that a recently introduced cementless TKA had results, both perioperatively and at an average of 2 years postoperatively, that were equivalent to those of its cemented predecessor, without any aseptic failures of either implant. Thus, this study justifies continued surveillance of this device to elucidate both its survivorship and if it can provide any long-term benefits.
Level of Evidence:
Therapeutic Level I. See Instructions for Authors for a complete description of levels of evidence.
Aseptic component loosening remains the most common indication for revision total knee arthroplasty (TKA)1. Thus, despite the excellent survivorship and clinical outcomes of TKA2,3, component fixation remains a long-term concern. The number of primary TKAs performed annually in the United States is increasing at an exponential rate, with an increasing percentage of younger patients seeking TKA4. The rate of aseptic component loosening is known to be greater in younger patients5,6; thus, the potential impact of this growing demographic on the rate of aseptic component loosening is a concern.
The optimal mode of fixation in TKA has been an area of debate for decades. Cementless prostheses remain an intriguing option because of the potential for biologic fixation and improved survivorship7. However, numerous prior reports of cementless TKA designs have raised concerns regarding failure of fixation, early failure, and poor clinical outcomes7-11. Furthermore, the immediate fixation and excellent survivorship of cemented TKA make transitioning from this technique difficult for the majority of surgeons11. Lastly, cementless prostheses are typically more expensive than their cemented counterparts, which can impact a surgeon’s choice of the mode of fixation.
Most early iterations of cementless implants had numerous design flaws, including the use of sintered beads or mesh coating, non-continuous fixation surfaces, poor polyethylene locking mechanisms and sterilization methods, and the use of metal-backed patellae known to have poor survivorship12. However, the clinical success of highly porous surfaces in total hip arthroplasty has stimulated an increased interest in their application to cementless TKA designs13. Numerous studies have demonstrated encouraging results with the use of modern cementless designs13-17. However, despite the use of highly porous surfaces, concerns about suboptimal fixation and worse clinical outcomes remain. Furthermore, not all implants with highly porous surfaces are the same, as numerous factors such as implant metallurgy, stiffness, surface coatings, and keel or peg fixation design can greatly influence outcomes. It remains necessary to analyze the results of recently introduced prosthetic designs to determine if poor outcomes would warrant their discontinuation.
Recently, a cementless TKA implant was introduced with design features similar to those of its cemented predecessor, but it has a highly porous titanium coating applied by 3-dimensional printing to encourage biologic fixation of the tibial component. The purpose of this prospective, randomized study was to determine if there are any differences in perioperative variables or clinical or radiographic outcomes between cemented and cementless TKAs of the same design.
Materials and Methods
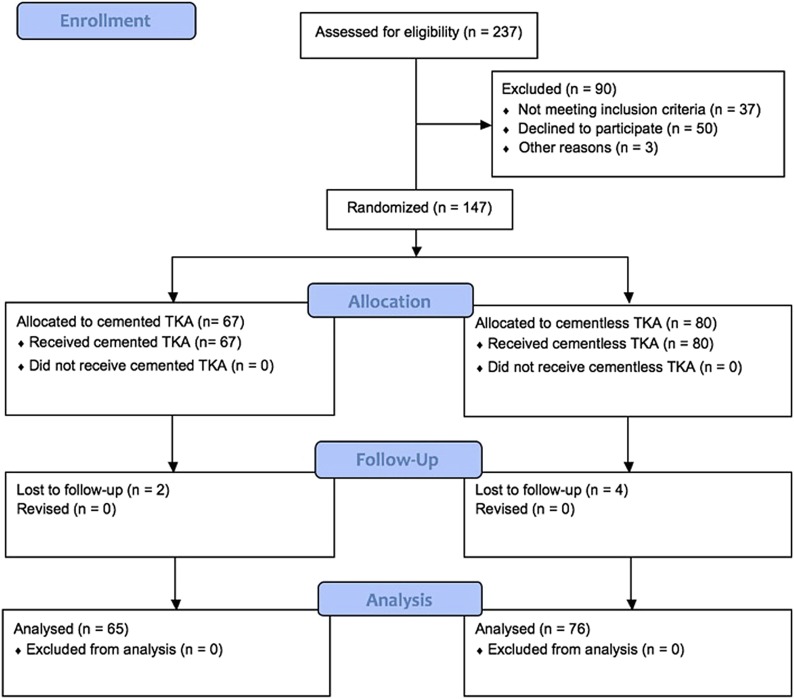
This study was an institutional review board-approved, prospective, randomized trial performed at a single academic institution and registered in ClinicalTrials.gov (identifier: NCT03683992). Inclusion criteria were an age between 18 and 75 years, a primary TKA for a diagnosis of arthritis, and the patient’s willingness to be randomized to be treated with a cemented or cementless TKA implant. Exclusion criteria were a diagnosis of inflammatory arthritis, a body mass index (BMI) of >40 kg/m2, active or suspected infection in the joint or body, prior fracture of the knee (patella, femur, or tibia), prior open surgery of the knee, a neuromuscular disorder, or grossly osteoporotic bone or bone defects seen on preoperative radiographs. From February 2014 to November 2016, patients meeting these criteria were randomized using computer-generated sequencing to receive either a cemented or a cementless cruciate-retaining Triathlon TKA implant (Stryker) (Fig. 1). Randomization and consent were overseen by a dedicated study coordinator. Four fellowship-trained total joint arthroplasty surgeons performed all TKAs. Each surgeon had a 1:1 block-randomization table with random block sizes to ensure similar group sizes for each surgeon while maintaining unpredictability of the randomization scheme. Baseline demographics were recorded.
Fig. 1.
Flow diagram demonstrating enrollment in the cemented and cementless cohorts.
Perioperative protocols were the same for all patients enrolled in this investigation. Patients received a multimodal pain management regimen that included use of a regional anesthetic and periarticular injection. Patients received 1 g of intravenous tranexamic acid (TXA) at the time of incision and at the start of wound closure18. Patients with a history of thromboembolic disease received 2 g of intra-articular TXA. Intra-articular drains were not used. A pneumatic thigh tourniquet was used only for patients receiving a cemented prosthesis. Exsanguination with an Esmarch bandage was performed prior to the skin incision; then the tourniquet was deflated after cementation of the prosthesis and prior to wound closure. The total operative time (from incision start to wound closure), estimated blood loss based on the anesthesia record, and change in hemoglobin level (g/dL) from preoperatively to the morning of postoperative day 1 were recorded.
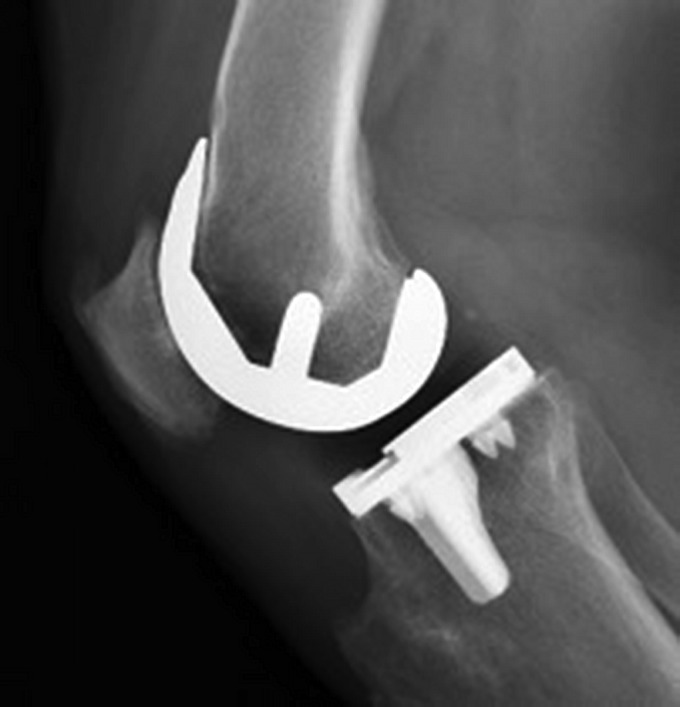
All patients received a cruciate-retaining prosthesis. In the cemented cohort, the femoral and tibial components were both fixed utilizing Simplex bone cement (Stryker) (Figs. 2-A and 2-B). The cementless prosthesis (Figs. 3-A and 3-B) consists of a beaded, Peri-Apatite-coated (Stryker) femoral component that incorporates multiple layers of cobalt-chromium beads and has a porosity of 40% and a mean pore size of 0.45 mm as measured by mean intercept length, creating a 3-dimensional (3-D) surface19. Medial and lateral distal pegs are present in this cruciate-retaining design for additional stability. The cementless tibial component (Triathlon Tritanium tibial baseplate; Stryker) has a highly porous titanium coating applied by 3-D printing to create a biologic fixation surface. A delta-shaped (triangular) keel and 4 cruciform pegs coated solely at the base of each peg are used for fixation19. Patellar resurfacing was not performed in either cohort.
Figs. 2-A and 2-B Anteroposterior (Fig. 2-A) and lateral (Fig. 2-B) radiographs demonstrating an implanted cemented prosthesis.
Fig. 2-A.
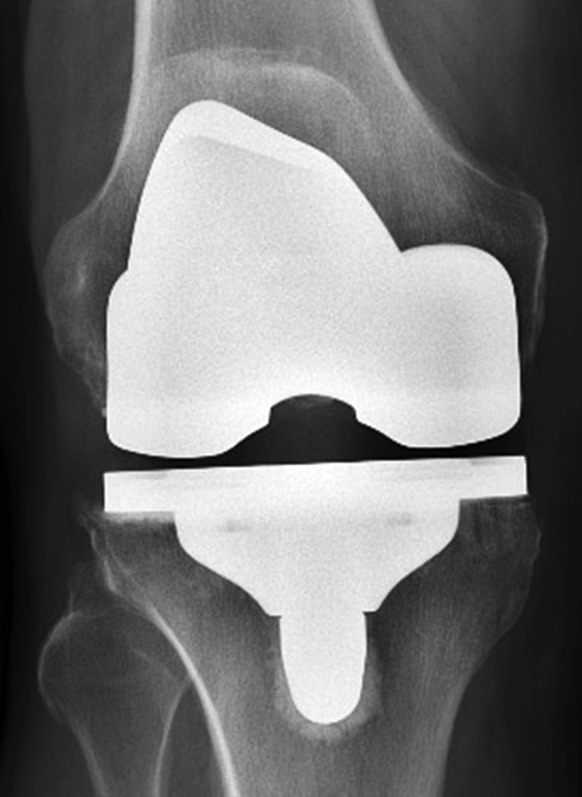
Fig. 2-B.
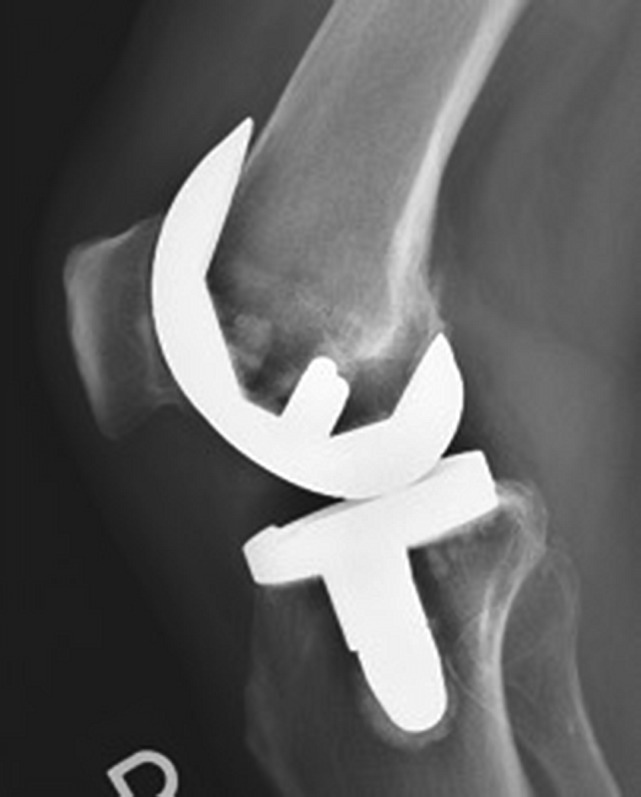
Figs. 3-A and 3-B Anteroposterior (Fig. 3-A) and lateral (Fig. 3-B) radiographs demonstrating an implanted cementless prosthesis.
Fig. 3-A.
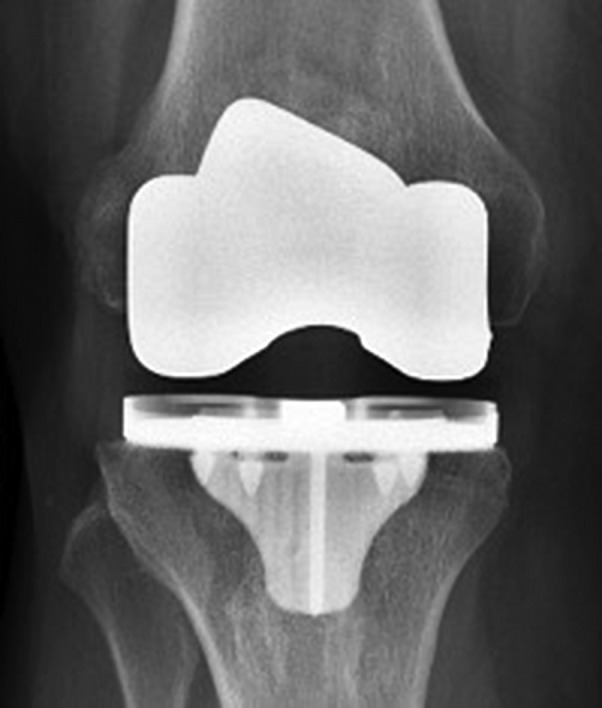
Fig. 3-B.
The primary outcome was the Oxford Knee Score20, which was collected preoperatively, at 4 to 6 weeks, at 1 year, and at an average of 2 years postoperatively. It should be noted that the original primary outcome, as listed in ClinicalTrials.gov, was “total tourniquet time,” but this could not be used when we elected not to utilize tourniquets in the cementless cohort.
Secondary outcome measures included the Knee Society Score21 and the Forgotten Joint Score22 (a measurement of a patient’s ability to forget about the joint as a result of surgical treatment), which were also collected at 4 to 6 weeks, 1 year, and an average of 2 years postoperatively. At 4 to 6 weeks postoperatively, the patients were also asked to grade their pain using a visual analog scale (VAS) ranging from 1 to 5, with 5 being “pain that wakes you up at night, or pain all the time.”23 In addition, patients were asked to grade their knee with the TKA as a percentage of “normal” (maximum, 100% [equivalent to completely normal]) at all 3 time points and rate their overall health (maximum, 100 [equivalent to the best possible health state]) and describe their satisfaction with the overall function of their TKA (extremely satisfied, very satisfied, quite satisfied, somewhat satisfied, not satisfied, or uncertain) at an average of 2 years. Several secondary outcome measures (University of California Los Angeles [UCLA] Activity Score, Short Form-12, and EuroQol-5 Dimensions Questionnaire) that were listed in ClinicalTrials.gov were ultimately not used in the study as we were concerned about fatiguing our patients—i.e., we thought that giving them too many surveys would limit their ability to answer all questions accurately.
Clinical radiographs were reviewed at 4 to 6 weeks, 1 year, and an average of 2 years postoperatively. Radiolucencies at the bone-implant interface were measured using the method described by Akizuki et al.24. The thickness of clear zones between the femoral and tibial implants and bone was measured in specific regions of the anteroposterior and lateral radiographs. Then the thicknesses in these regions were summed to calculate a total for each bone (the femur and tibia), and the total was divided by the number of regions to calculate the mean width of the clear zone in that bone.
Statistical Analysis
A power analysis demonstrated that a total sample size of 130 patients was needed to show a difference in the mean Oxford Knee Scores of 5 points at 90% power, accepting a type-I error rate of 5%. A 5-point difference in the Oxford Knee Score has previously been reported to be the minimal clinically important difference25. In order to account for potential noncompliance with follow-up, 15% was added to our sample size, for a total of 150 patients, and 237 patients (not a sequential series of cases) were approached for enrollment (Fig. 1). Ninety of these patients were not enrolled because they declined to participate (n = 50), because they did not meet inclusion criteria (n = 37), or for other reasons (n = 3). During the study period, the participating surgeons performed approximately 1,150 TKAs, and each screened their patients for eligibility for the investigation on the basis of exclusion criteria. If deemed suitable by the surgeon, the patient was asked by the surgeon if he or she would be willing to participate in a randomized controlled trial comparing cemented and cementless prostheses. Of these patients, 237 initially stated they were willing to participate and willing to discuss the investigation with the study coordinator, and 147 of them were enrolled. Patients who were unwilling to be part of this prospective, randomized controlled trial were still eligible to receive the cemented or cementless prosthesis at each surgeon’s discretion. The outcomes of these patients were retrospectively reviewed and have been previously published26.
Only as-treated analyses were conducted for all comparisons as there were no crossovers between the cemented and cementless cohorts requiring an intent-to-treat analysis. Independent-samples t tests were used to assess group differences in continuous variables, and Pearson chi-square tests were used to assess categorical variables. A p value of <0.05 was considered significant. All statistical analyses were performed using SPSS for Windows, version 22 (IBM).
Results
One hundred and forty-seven patients (67 cemented and 80 cementless prostheses) were enrolled in this prospective, randomized trial. One hundred and forty-one patients (96%) (65 cemented and 76 cementless prostheses) had complete clinical and radiographic follow-up at an average of 2 years postoperatively. There were no differences in baseline demographics or duration of follow-up between the cemented and cementless cohorts (Table I).
TABLE I.
Comparison of Baseline Demographics and Duration of Follow-up Between the Cemented and Cementless Cohorts
| Cemented (N = 65) | Cementless (N = 76) | P Value | |
| Age* (yr) | 63.0 ± 7.6 | 61.3 ± 7.0 | 0.1 |
| Sex (% female) | 52 | 48 | 0.1 |
| BMI* (kg/m2) | 31.3 ± 4.7 | 31.1 ± 5.2 | 0.8 |
| ASA score* | 2.1 ± 0.6 | 2.1 ± 0.5 | 0.9 |
| Duration of follow-up* (mo) | 24.9 ± 3.3 | 25.2 ± 3.9 | 0.6 |
The values are given as the mean and standard deviation. ASA = American Society of Anesthesiologists.
The mean total operative time (and standard deviation) in the cementless cohort was 82.1 ± 16.6 minutes, which was significantly less (p = 0.001) than that in the cementless cohort (93.7 ± 16.7 minutes), but there were no differences in perioperative blood loss (p = 0.9) or change in hemoglobin level from preoperatively to day 1 postoperatively (p = 0.5) between the 2 groups (Table II).
TABLE II.
Comparison of Intraoperative and Perioperative Variables Between the Cemented and Cementless Cohorts
| Cemented* (N = 65) | Cementless* (N = 76) | P Value† | |
| Operative time (min) | 93.7 ± 16.7 | 82.1 ± 16.6 | 0.001 |
| Estimated blood loss (mL) | 185.2 ± 134.9 | 183.3 ± 146.7 | 0.9 |
| Hemoglobin (g/dL) | |||
| Preoperative | 13.6 ± 1.3 | 14.2 ± 1.4 | 0.01 |
| Postoperative | 11.1 ± 1.2 | 11.6 ± 1.4 | 0.03 |
| Change | −2.5 ± 0.9 | −2.6 ± 1.4 | 0.5 |
The values are given as the mean and standard deviation.
Significant p values are noted in bold.
Both at 4 to 6 weeks and at 1 year postoperatively, there were no differences between the 2 cohorts in terms of the Oxford Knee Score, Knee Society Score, Forgotten Joint Score, or rating of the knee with the TKA as a percentage of “normal” (p = 0.1 to 0.9) (Tables III, IV, and V). Of note, at 4 to 6 weeks postoperatively, there was also no difference in the percentage of patients who reported “no pain” (31% in the cemented group and 34% in the cementless group, p = 0.7) or in the mean VAS score (Table III). At 1 year postoperatively, no TKAs in either cohort required a revision surgical procedure and there were no radiographic findings of component subsidence or failure.
TABLE III.
Comparison of Pain Scores Between the Cemented and Cementless Cohorts at 4 to 6 Weeks Postoperatively
| Cemented (N = 65) | Cementless (N = 76) | P Value | |
| No pain (%) | 31 | 34 | 0.7 |
| VAS score* (1-5) | 3.5 ± 1.4 | 3.2 ± 1.1 | 0.3 |
The values are given as the mean and standard deviation.
TABLE IV.
Comparison of Clinical Outcome Scores Between the Cemented and Cementless Cohorts at 4 to 6 Weeks Postoperatively
| Cemented* (N = 65) | Cementless* (N = 76) | P Value | |
| Oxford Knee Score | |||
| Preoperative | 23.6 ± 6.5 | 21.5 ± 8.1 | 0.1 |
| Postoperative | 24.5 ± 8.9 | 23.8 ± 8.9 | 0.3 |
| Change | 0.9 ± 9.1 | 2.1 ± 9.2 | 0.4 |
| Knee Society Score | |||
| Preoperative | 43.2 ± 13.6 | 39.3 ± 16.6 | 0.2 |
| Postoperative | 41.7 ± 18.0 | 41.4 ± 17.3 | 0.9 |
| Change | −1.3 ± 19.2 | 1.8 ± 18.1 | 0.3 |
| Forgotten Joint Score | 24.1 ± 22.5 | 24.1 ± 26.0 | >0.9 |
| % of normal knee | 65.1 ± 19.0 | 64.2 ± 18.0 | 0.8 |
The values are given as the mean and standard deviation.
TABLE V.
Comparison of Clinical Outcome Scores Between the Cemented and Cementless Cohorts at 1 Year Postoperatively
| Cemented* (N = 65) | Cementless* (N = 76) | P Value | |
| Oxford Knee Score | |||
| Preoperative | 23.6 ± 6.5 | 21.5 ± 8.1 | 0.1 |
| Postoperative | 37.4 ± 10.4 | 39.3 ± 8.7 | 0.3 |
| Change | 14.5 ± 11.2 | 17.4 ± 9.4 | 0.1 |
| Knee Society Score | |||
| Preoperative | 43.2 ± 13.6 | 39.3 ± 16.6 | 0.2 |
| Postoperative | 72.4 ± 16.3 | 76.7 ± 19.1 | 0.2 |
| Change | 31.3 ± 18.5 | 35.6 ± 19.8 | 0.2 |
| Forgotten Joint Score | 58.5 ± 25.5 | 60.5 ± 25.1 | 0.6 |
| % of normal knee | 85.1 ± 15.7 | 88.7 ± 10.2 | 0.1 |
The values are given as the mean and standard deviation.
At an average of 2 years postoperatively, there were again no differences in any clinical outcome measure between the 2 groups (Table VI). Approximately 68% of the patients in the cemented cohort and 75% in the cementless cohort were “extremely” or “very” satisfied with the function of their knee (p = 0.7). Patients in the cemented cohort rated their knee to be 88.2% ± 12.0% of “normal” compared with 87.4% ± 14.5% in the cementless cohort (p = 0.7). Similarly, there was no difference in the postoperative overall health rating between the 2 groups (81.1 ± 13.9 and 82.3 ± 13.9, p = 0.6).
TABLE VI.
Comparison of Clinical Outcome Scores Between the Cemented and Cementless Cohorts at 2 Years Postoperatively
| Cemented (N = 65) | Cementless (N = 76) | P Value | |
| Oxford Knee Score* | |||
| Preoperative | 23.6 ± 6.5 | 21.5 ± 8.1 | 0.1 |
| Postoperative | 39.6 ± 9.1 | 41.0 ± 7.5 | 0.3 |
| Change | 17.3 ± 10.5 | 19.7 ± 8.7 | 0.2 |
| Knee Society Score* | |||
| Preoperative | 43.2 ± 13.6 | 39.3 ± 16.6 | 0.2 |
| Postoperative | 75.6 ± 17.9 | 78.5 ± 17.5 | 0.3 |
| Change | 33.5 ± 19.7 | 39.2 ± 25.2 | 0.2 |
| Forgotten Joint Score* | 66.6 ± 33.0 | 61.5 ± 31.1 | 0.3 |
| % of normal knee* | 88.2 ± 12.0 | 87.4 ± 14.5 | 0.7 |
| Overall health rating* | |||
| Preoperative | 74.5 ± 17.0 | 74.4 ± 15.3 | 0.9 |
| Postoperative | 81.1 ± 13.9 | 82.3 ± 13.9 | 0.6 |
| Change | 7.0 ± 19.9 | 8.8 ± 19.2 | 0.6 |
| Satisfaction with overall function (%) | 0.7 | ||
| Extremely | 37 | 41 | |
| Very | 31 | 34 | |
| Quite | 11 | 5 | |
| Somewhat | 6 | 5 | |
| Slightly | 0 | 3 | |
| Not | 6 | 4 | |
| Uncertain | 9 | 8 |
The values are given as the mean and standard deviation.
Radiographic analysis showed no progressive radiolucencies or signs of component subsidence or failure at an average of 2 years postoperatively. The mean thickness of the clear zones around the tibial component was 0.01 ± 0.01 mm in the cemented cohort compared with 0.02 ± 0.03 mm in the cementless cohort (p = 0.01). The mean thicknesses of the clear zones around the femoral component were 0.01 ± 0.02 and 0.02 ± 0.02 mm, respectively (p = 0.01). One revision procedure was performed in the cemented cohort for periprosthetic infection whereas no revisions were performed in the cementless cohort.
Discussion
Aseptic loosening accounts for 31% to 39% of indications for revision TKA1,27. Thus, methods to potentially improve implant survivorship continue to be investigated. Prior iterations of cementless implants had numerous design flaws contributing to the increased failure rates seen with their use compared with their cemented counterparts7-11. The advent of highly porous surfaces with properties resembling trabecular bone has shown promising early results13,16,17,28. However, concerns about failure of fixation with cementless knee prostheses remain. The purpose of this study was to compare a recently introduced cementless TKA with its cemented predecessor. At an average of 2 years postoperatively, nearly identical clinical results were achieved using this cementless design, without any cases of aseptic failure. Continued surveillance is necessary to determine the potential long-term benefits of this cementless design.
Cementation continues to be the favored mode of fixation in TKA by the majority of surgeons as it has demonstrated excellent survivorship and clinical function2,3. However, the benefit of initial, rigid fixation is mitigated by cement’s poor resistance to shear and tensile forces, which can eventually result in micromotion and component loosening19. Cementless fixation eliminates the risk of cement particles and decreases the risk of third-body debris, while potentially forming a biologic interface that can remodel and adapt over time12,24.
Numerous investigators have reported promising early results with the use of modern iterations of cementless TKA incorporating highly porous surfaces16,17,28,29, with most of these authors describing the use of Trabecular Metal (Zimmer Biomet), or tantalum, as the biologic interface. DeFrancesco et al. reported excellent results with use of a cementless, tantalum monoblock tibial component in patients <60 years of age, noting no revisions related to tibial fixation and an all-cause revision rate of 6% at 10 years postoperatively30. Fricka et al. performed a prospective, randomized trial of 100 TKAs comparing cemented and cementless prostheses that had a modular, Trabecular Metal tibial tray design16. They found higher Knee Society Scores in the cemented cohort (96.4 compared with 92.3, p = 0.03) and a higher rate of radiolucencies in the cementless cohort. Furthermore, there were 4 cases of varus tibial subsidence in the cementless cohort, with a mean change in position of 3° at 2 years postoperatively. Although these components were believed to stabilize, continued follow-up is necessary to determine their long-term stability and function. Thus, the results of this prior investigation were not as favorable for that particular cementless design.
Unfortunately, there have been a number of recent implant recalls and prosthetic failures in the field of arthroplasty. Thus, it is critical to study any new prosthesis for early radiographic or clinical signs of potential failure. Furthermore, given the vast number of variables, such as implant metallurgy, surface coating, and fixation design, that can have substantial ramifications with regard to patient outcomes, it is clear that not all cementless designs are equivalent. This investigation focused on a recently introduced cementless design with characteristics similar to those of its cemented predecessor28. To our knowledge, this is the first prospective, randomized investigation of this implant design, and it showed no difference in clinical outcomes between the cemented and cementless cohorts as well as excellent results without failure for aseptic loosening at 2 years postoperatively in both groups. Furthermore, no clinical differences between the 2 cohorts were appreciated at any time following implantation, starting as early as 4 to 6 weeks postoperatively. The mean VAS score and the percentage of patients reporting no pain at 4 to 6 weeks were the same in the 2 cohorts. Thus, the concern about potentially increased pain during the early period, prior to biologic fixation, after cementless TKA was not borne out by this investigation. However, given the increased cost of cementless implant designs, the burden of proof remains with cementless fixation—i.e., it must be shown to be superior to cement fixation. Thus, despite the encouraging results found with this cementless prosthesis, continued surveillance is necessary to determine the potential long-term benefits of this design.
This study has several limitations. First, only 4 surgeons from a single tertiary care center enrolled patients in this investigation. Thus, these results may not be generalizable to other centers. In addition, patients deemed to have osseous defects or severe osteoporosis were excluded prior to enrollment at each surgeon’s discretion. Therefore, as there were strict inclusion and exclusion criteria for study eligibility, this study’s results should not be misinterpreted as indicating that all patients are candidates for cementless fixation. In addition, it is important to note that, because of these exclusion criteria and the fact that many patients were unwilling to participate, the patients enrolled in this investigation represented a minority of all TKAs performed during the study period. Therefore, the generalizability of our results to other patient populations with different demographics is limited. Third, the duration of follow-up was short. Continued follow-up is necessary to ensure that the clinical outcomes do not worsen, and differences between the 2 cohorts do not become apparent, over time. However, given the recent introduction of this specific cementless design, we thought that it was critical to evaluate this device at early time points to ensure that abnormal rates of failure or poor clinical performance were not being overlooked.
Conclusions
This study demonstrated that the results of a recently introduced cementless TKA were equivalent to those of its cemented predecessor, without aseptic failure in either group, both perioperatively and at an average of 2 years postoperatively. Thus, this study justifies continued surveillance of this device to elucidate both its survivorship and whether it has any long-term benefits.
Footnotes
Investigation performed at Washington University School of Medicine, St. Louis, Missouri
Disclosure: Research support was provided to the investigating institution by Stryker Inc., the manufacturer of the implant under investigation. On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one or more of the authors checked “yes” to indicate that the author had a relevant financial relationship in the biomedical arena outside the submitted work (http://links.lww.com/JBJS/F254).
Data Sharing
A data-sharing statement is provided with the online version of the article (http://links.lww.com/JBJS/F380).
References
- 1.Sharkey PF, Lichstein PM, Shen C, Tokarski AT, Parvizi J. Why are total knee arthroplasties failing today—has anything changed after 10 years? J Arthroplasty. 2014. September;29(9):1774-8. Epub 2014 Jul 5. [DOI] [PubMed] [Google Scholar]
- 2.Australian Orthopaedic Association National Joint Replacement Registry annual report. https://aoanjrr.dmac.adelaide.edu.au/en. Accessed 2014 Apr 20. [DOI] [PubMed]
- 3.New Zealand National Joint Registry 10 year report. http://www.nzoa.org.nz/nz-joint-registry. Accessed 2014 Apr 20.
- 4.Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res. 2009. October;467(10):2606-12. Epub 2009 Apr 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr AJ, Robertsson O, Graves S, Price AJ, Arden NK, Judge A, Beard DJ. Knee replacement. Lancet. 2012. April 7;379(9823):1331-40. Epub 2012 Mar 6. [DOI] [PubMed] [Google Scholar]
- 6.Julin J, Jämsen E, Puolakka T, Konttinen YT, Moilanen T. Younger age increases the risk of early prosthesis failure following primary total knee replacement for osteoarthritis. A follow-up study of 32,019 total knee replacements in the Finnish Arthroplasty Register. Acta Orthop. 2010. August;81(4):413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lombardi AV, Jr, Berasi CC, Berend KR. Evolution of tibial fixation in total knee arthroplasty. J Arthroplasty. 2007. June;22(4)(Suppl 1):25-9. [DOI] [PubMed] [Google Scholar]
- 8.Illgen R, Tueting J, Enright T, Schreibman K, McBeath A, Heiner J. Hybrid total knee arthroplasty: a retrospective analysis of clinical and radiographic outcomes at average 10 years follow-up. J Arthroplasty. 2004. October;19(7)(Suppl 2):95-100. [DOI] [PubMed] [Google Scholar]
- 9.Parker DA, Rorabeck CH, Bourne RB. Long-term followup of cementless versus hybrid fixation for total knee arthroplasty. Clin Orthop Relat Res. 2001. July;388:68-76. [DOI] [PubMed] [Google Scholar]
- 10.Rand JA, Trousdale RT, Ilstrup DM, Harmsen WS. Factors affecting the durability of primary total knee prostheses. J Bone Joint Surg Am. 2003. February;85(2):259-65. [DOI] [PubMed] [Google Scholar]
- 11.Robertsson O, Bizjajeva S, Fenstad AM, Furnes O, Lidgren L, Mehnert F, Odgaard A, Pedersen AB, Havelin LI. Knee arthroplasty in Denmark, Norway and Sweden. A pilot study from the Nordic Arthroplasty Register Association. Acta Orthop. 2010. February;81(1):82-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cherian JJ, Banerjee S, Kapadia BH, Jauregui JJ, Harwin SF, Mont MA. Cementless total knee arthroplasty: a review. J Knee Surg. 2014. June;27(3):193-7. Epub 2014 Apr 24. [DOI] [PubMed] [Google Scholar]
- 13.Kamath AF, Lee GC, Sheth NP, Nelson CL, Garino JP, Israelite CL. Prospective results of uncemented tantalum monoblock tibia in total knee arthroplasty: minimum 5-year follow-up in patients younger than 55 years. J Arthroplasty. 2011. December;26(8):1390-5. Epub 2011 Aug 26. [DOI] [PubMed] [Google Scholar]
- 14.Unger AS, Duggan JP. Midterm results of a porous tantalum monoblock tibia component clinical and radiographic results of 108 knees. J Arthroplasty. 2011. September;26(6):855-60. Epub 2010 Oct 29. [DOI] [PubMed] [Google Scholar]
- 15.Park JW, Kim YH. Simultaneous cemented and cementless total knee replacement in the same patients: a prospective comparison of long-term outcomes using an identical design of NexGen prosthesis. J Bone Joint Surg Br. 2011. November;93(11):1479-86. [DOI] [PubMed] [Google Scholar]
- 16.Fricka KB, Sritulanondha S, McAsey CJ. To cement or not? Two-year results of a prospective, randomized study comparing cemented vs. cementless total knee arthroplasty (TKA). J Arthroplasty. 2015. September;30(9)(Suppl):55-8. Epub 2015 Jun 3. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Fairen M, Hernández-Vaquero D, Murcia A, Torres A, Llopis R. Trabecular Metal in total knee arthroplasty associated with higher knee scores: a randomized controlled trial. Clin Orthop Relat Res. 2013. November;471(11):3543-53. Epub 2013 Jul 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillette BP, DeSimone LJ, Trousdale RT, Pagnano MW, Sierra RJ. Low risk of thromboembolic complications with tranexamic acid after primary total hip and knee arthroplasty. Clin Orthop Relat Res. 2013. January;471(1):150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harwin SF, Elmallah RK, Jauregui JJ, Cherian JJ, Mont MA. Outcomes of a newer-generation cementless total knee arthroplasty design. Orthopedics. 2015. October;38(10):620-4. [DOI] [PubMed] [Google Scholar]
- 20.Dawson J, Fitzpatrick R, Carr A. Questionnaire on the perceptions of patients about shoulder surgery. J Bone Joint Surg Br. 1996. July;78(4):593-600. [PubMed] [Google Scholar]
- 21.Scuderi GR, Bourne RB, Noble PC, Benjamin JB, Lonner JH, Scott WN. The new Knee Society Knee Scoring System. Clin Orthop Relat Res. 2012. January;470(1):3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Behrend H, Giesinger K, Giesinger JM, Kuster MS. The “forgotten joint” as the ultimate goal in joint arthroplasty: validation of a new patient-reported outcome measure. J Arthroplasty. 2012. March;27(3):430-436.e1. Epub 2011 Oct 13. [DOI] [PubMed] [Google Scholar]
- 23.Nam D, Nunley RM, Sauber TJ, Johnson SR, Brooks PJ, Barrack RL. Incidence and location of pain in young, active patients following hip arthroplasty. J Arthroplasty. 2015. November;30(11):1971-5. Epub 2015 May 23. [DOI] [PubMed] [Google Scholar]
- 24.Akizuki S, Takizawa T, Horiuchi H. Fixation of a hydroxyapatite-tricalcium phosphate-coated cementless knee prosthesis. Clinical and radiographic evaluation seven years after surgery. J Bone Joint Surg Br. 2003. November;85(8):1123-7. [DOI] [PubMed] [Google Scholar]
- 25.Clement ND, MacDonald D, Simpson AH. The minimal clinically important difference in the Oxford Knee Score and Short Form 12 score after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2014. August;22(8):1933-9. Epub 2013 Nov 20. [DOI] [PubMed] [Google Scholar]
- 26.Nam D, Kopinski JE, Meyer Z, Rames RD, Nunley RM, Barrack RL. Perioperative and early postoperative comparison of a modern cemented and cementless total knee arthroplasty of the same design. J Arthroplasty. 2017. July;32(7):2151-5. Epub 2017 Feb 7. [DOI] [PubMed] [Google Scholar]
- 27.Schroer WC, Berend KR, Lombardi AV, Barnes CL, Bolognesi MP, Berend ME, Ritter MA, Nunley RM. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013. September;28(8)(Suppl):116-9. Epub 2013 Aug 15. [DOI] [PubMed] [Google Scholar]
- 28.Harwin SF, Patel NK, Chughtai M, Khlopas A, Ramkumar PN, Roche M, Mont MA. Outcomes of newer generation cementless total knee arthroplasty: beaded periapatite-coated vs highly porous titanium-coated implants. J Arthroplasty. 2017. July;32(7):2156-60. Epub 2017 Feb 3. [DOI] [PubMed] [Google Scholar]
- 29.Hayakawa K, Date H, Tsujimura S, Nojiri S, Yamada H, Nakagawa K. Mid-term results of total knee arthroplasty with a porous tantalum monoblock tibial component. Knee. 2014. January;21(1):199-203. Epub 2013 Jul 18. [DOI] [PubMed] [Google Scholar]
- 30.DeFrancesco CJ, Canseco JA, Nelson CL, Israelite CL, Kamath AF. Uncemented tantalum monoblock tibial fixation for total knee arthroplasty in patients less than 60 years of age: mean 10-year follow-up. J Bone Joint Surg Am. 2018. May 16;100(10):865-70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data-sharing statement is provided with the online version of the article (http://links.lww.com/JBJS/F380).