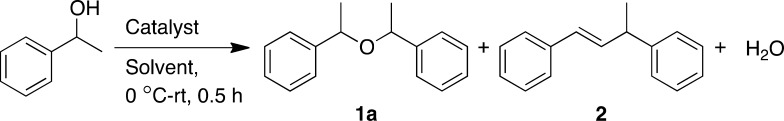
Table 1. Synthesis of Symmetrical Ethers: Optimization of Reaction Conditionsa.
| entry | catalyst (mol %) | solvent | yield of 1a/2 (%)b |
|---|---|---|---|
| 1 | FeCl3·6H2O (5) | DCM | 65/15 |
| 2 | Fe(NO3)3 (5) | DCM | 0 |
| 3 | Fe(OTf)3 (5) | DCM | 10/70 |
| 4 | Fe(OTf)3 (5) + NH4Cl (5) | DCM | 81/0 |
| 5 | Fe(OTf)3 (5) + NH4Cl (20) | DCM | 80/0 |
| 6 | Fe(OTf)3 (5) + NH4Cl (5) | CH3CN | trace |
| 7 | Fe(OTf)3 (5) + NH4Cl (5) | THF | 0 |
| 8 | Fe(OTf)3 (5) + NH4Cl (5) | acetone | 0 |
| 9 | Fe(OTf)3 (5) + NH4Cl (5) | toluene | 0 |
| 10 | DCM | 0 | |
| 11 | NH4Cl (5) | DCM | 0 |
Reaction conditions: 1-phenylethanol (0.5 mmol), solvent (2 mL), Fe(OTf)3 (0.025 mmol, 5 mol %), and NH4Cl (0.025 mmol, 5 mol %) were stirred at 0 °C to room temperature (rt) for 0.5 h in open air.
Isolated yield.