Abstract
Actinic keratoses (AKs) are precancerous epidermal lesions that develop on sensitive, frequently sun-exposed skin surfaces. There are very little data regarding AK prevalance. The aim of this study was to investigate the prevalence of AK and related conditions among patients in a dermatology outpatient clinic.
Patients attending our dermatology outpatient clinic between January 1, 2015 and December 31, 2017 were evaluated retrospectively usingan automated system. A total of 54,786 patients aged ≥30 years attending the dermatology outpatient clinic were included in the study. We identified 1375 patients diagnosed with AK.
In our study, the AK prevalence was 0.01% for patients between 30 and 39 years of age, 0.45% for patients between 40 and 49 years of age, 1.77% for patients between 50 and 59 years of age, 4.61% for patients between 60 and 69 years of age, 9.38% for patients between 70 and 79 years of age, and 14.57% for patients ≥80 years. AK prevalence was 2.50% among patients of all ages.
The exposure to sunlight is excessive due to the geographical location of our country. Due to the tendency of AKs to convert to malignancies, the identification of patients at high risk for AK development and the identification of high-risk anatomical regions are important to establish the basis of effective screening programs to support public health.
Keywords: actinic keratosis, exposure to sunlight, precancerous
1. Introduction
Actinic keratoses (AKs) are precancerous epidermal lesions that develop in sensitive, frequently sun-exposed skin. AKs typically manifest as multiple, red, scaly patches involving sun-exposed areas of the skin.[1–3] The main risk factor for AK development is cumulative UV radiation exposure throughout one's lifetime. Age, sex, and skin phototype are other independent risk factors. AKs are more frequently located on the head and the upper extremities, where the degree of sun exposure is greatest.[1,2,4,5]
The presence of AK lesions is considered to be a predisposing factor for squamous cell carcinoma (SCC) and basal cell carcinoma (BCC).[1–3,6] To prevent this transformation, AKs should be treated regardless of their stage.[4] The prevalence of AKs is therefore an important public health problem. There is very little data on the prevalence of AKs, and the data that are available have mostly originated from Australia and the United States. The studies from the continents of Europe and Asia are very limited.[1–6]
The aim of this study was to retrospectively evaluate the patients diagnosed with AK in our dermatology outpatient clinic and to determine the frequency of AK. We also aimed to evaluate the relationships of AK with age, sex, residential region, skin type, socioeconomic status, frequency of sun exposure owing to occupational and daily activity, and history of skin cancer and to compare our findings with existing literature.
2. Materials and methods
Patients attending our dermatology outpatient clinic between January 1, 2015 and December 31, 2017 were evaluated retrospectively using an automated system. A total of 1,375 patients who were diagnosed with AK were included in the study by considering the initial patient attendances. Lesions with an atypical appearance that were being biopsied were excluded from the study. The ages, sexes, skin types, socioeconomic statuses, frequencies of sun exposure owing to occupational and daily activity, lesion locations, and lesion numbers of AK patients were recorded.
Patients were classified into 7 age groups (under 30 years of age, 30–39, 40–49, 50–59, 60–69, 70–79, and ≥80 years) 4 skin type groups according to the Fitzpatrick skin classification, 3 socioeconomic groups (low, medium, and high), 3 sun exposure groups (rare = <2 days per week, frequent = 2–5 days per week, very frequent = >5 days per week), 2 lesion location groups (head or head and extremity), and 2 total lesion number groups (<5 and ≥5).
2.1. Statistics
The χ2 test and the IBM SPSS version 21 computer program were used for statistical analysis. The χ2 test and t test were used for comparison between groups. Pearson test was used to evaluate correlation. In all analyses, P < .05 was considered statistically significant.
3. Results
3.1. Patient characteristics
A total of 1375 patients were included in the study. Of these patients, 758 (55.12%) were males, and 617 (44.87%) were females. The mean age of all patients was 69.55 (range: 23–101) years. The mean age of female patients was 69.55 (range: 23–95) years, and the mean age of male patients was 69.56 (range: 26–101) years (Table 1, Fig. 1).
Table 1.
Socioeconomic status of patients, Fitzpatrick skin type, sun exposure, distribution of actinic keratosis according to the body regions.

Figure 1.
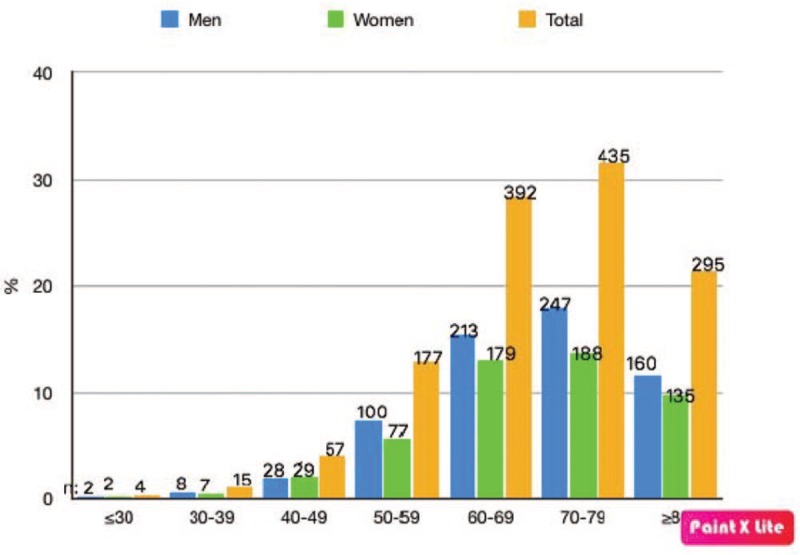
Distribution of patients included in the study according to age.
AKs were most common in patients 70 to 79 years of age, of which there were 435 (31%, 63%). AKs were least common in patients under 30 years of age, of which there were 4 (0.30%). There was a statistically significant correlation between patient age and AK prevalence (P < .001) and a statistically insignificant correlation between male sex and AK prevalence (P = .513).
3.2. AK prevalence
A total of 54,786 patients aged ≥30 years attending the dermatology outpatient clinic were included in the study. Among these patients, we identified 1375 with AK. In our study, AK prevalence was determined as 0.01% for patients between 30 and 39 years of age, 0.45% for patients between 40 and 49 years of age, 1.77% for patients between 50 and 59 years of age, 4.61% for patients between 60 and 69 years of age, 9.38% for patients between 70 and 79 years of age, and 14.57% for patients ≥80 years. AK prevalence was determined as 2.50% among patients of all ages (Table 2).
Table 2.
Prevalence of patients according to age.

3.3. Background
The skin cancer history of all patients included in the study was evaluated. Among males, 51 (3.71%) had a history of SCC, and 84 (6.1%) had a history of BCC. Among females, 46 (3.34%) had a history of SCC, and 62 (4.50%) had a history of BCC. A total of 243 (17.67%) patients with AK had a history of either BCC (10.61%) or SCC (7.05%).
3.4. Extent of disease
The number of AK lesions was recorded for all patients included in the study. A high AK load was defined as >5 AK lesions appearing in 1 patient. There were ≥5 lesions in 993 (72.22%) patients and <5 in 382 (27.78%) patients. In 2 different anatomical regions, AKs were more common in females (52.5%) than in males (P < .001) (Table 1). The elevated prevalence of AK in both sexes >60 years was statistically significant (P < .001). Among the patients, 8% had <5 lesions, and 23.64% had ≥5 lesions, and lesions were most common in patients aged 70 to 80 years (P < .001). There was a correlation between the number of lesions, age, and sun exposure (P = .026, t = 0.244; P < .001, t = 0.398). However, no significant correlation was found between the number of lesions and sex (P = .833) (Table 3).
Table 3.
Statistical evaluation of number of lesions according to sex, age, income level, Fitzpatrick skin type, anatomical localization, sun exposure.
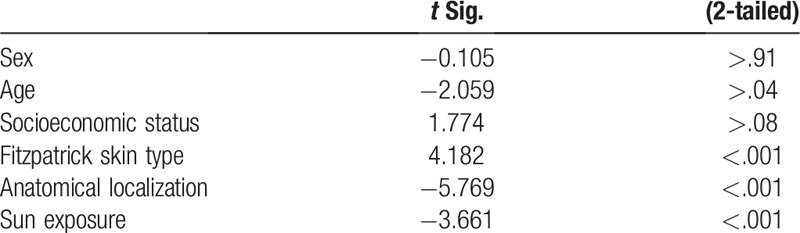
3.5. AK lesion localization
The lesions were found only on the head in 47.50% of female patients and in the region of the head, arms, and hands in 52.50% of female patients. They were found only on the head in 57.78% of males and in the region of the head, arms, and hands in 42.22% of males (Table 1).
In male patients aged 50 to 59 years, all lesions were seen only in the head region. In male patients aged 60 to 69 years, lesions were found only in the head region in 27.90% of patients and on the head, arms, and hands in 72.10% of patients. In male patients aged 70 to 79 years of age and aged ≥80 years, lesions were found at the rates of 22.22% and 77.78%, respectively.
In female patients aged 50 to 59 years, AKs were seen only in the head region in 25.80% of patients and on the head, arms, and hands in 74.20% of patients. In female patients aged 70 to 80 years and aged ≥80 years, lesions were found at the rates of 12.50% and 87.50%, respectively.
3.6. UV exposure among AK patients
When the patients were evaluated in terms of the incidence of sun exposure, very frequent exposure rates were found in 40% in males and 57.50% of females (Table 1). Whereas sun exposure was very frequent in 33.33%, frequent in 24.44%, and rare in 13.33% of males with ≥5 AK lesions, it was very frequent in 50.23%, frequent in 15.61%, and rare in 10.38% of females. The relationship between sun exposure and lesion location and number was statistically significant (P = .01).
3.7. Skin type among AK patients
The skin types of patients were classified according to Fitzpatrick skin type (type 1, 2, 3, or 4) (Table 1). In male patients, ≥5 lesions were seen in all patients with Fitzpatrick skin type 1 (100%), in 85.71% of patients with skin type 2, and in 52.38% of patients with skin type 3. None of the male patients with skin types 4 and 5 had ≥5. In females, ≥5 lesions were seen in 65.96% of patients with skin type 1, in 78.57% of patients with skin type 2, and in 46.12% of patients with skin type 3. The distribution of patients with AK in both sexes was statistically significant (P < .001). In this distribution, the most common lesion was seen as type 2, and the least common lesion was seen as type 4.
3.8. Economic status among AK patients
Patients in this study were divvied into 3 economic status groups: low, medium, and high. The middle-income level was identified as the most common group in both sexes. The high income level was the least common group in males (4.44%). In females, there were no patients in the high-income group (Table 1).
4. Discussion
In this study, age, sex, skin type, socioeconomic status, lesion localization and number, and UV exposure of patients with AK from my outpatient clinic were evaluated, as well as the relationships among these factors. In our study, AK prevalence was higher in patients >60 years of age and reached the maximum level in patients >80 years of age. AK was more prevalent in males and in patients with Fitzpatrick skin types 2 and 3. The number of lesions increased and affected >1 region as UV exposure increased. In addition, the prevalence of AK was 2.5% among the patients attending the dermatology outpatient clinic. To the best of our knowledge, there have been no previous detailed studies of AK prevalence in our country. There are relatively few studies on AK prevalence in the literature.[4,7–10] In previous studies, the incidence of AK in patients attending the dermatology outpatient clinic was reported to be between 5% and 30%.[11–13] Dziunycz et al[4] reported AK frequency as 25.3% in general population, 6.5% among individuals 30 to 40 years of age, and 69.4% in individuals 90 to 100 years of age. Eder et al[11] found an AK prevalence of 1.1% in females between 30 and 39 years of age, 4% in males between 60 and 70 years of age, 23.1% in females of all ages, 39% in males of all ages, 68.1% in females ≥90 years, and 89.7% in males ≥90 years. Memon et al[14] reported an AK prevalence of 15.4% in males between 40 and 70 years of age, 5.9% in females between 40 and 70 years of age, 34.1% in males >70 years, and 18% in females >70 years. Naldi et al[15] and Harver et al[16] reported an AK prevalence of between 1.4% and 16% in persons >45 years in their studies in different regions. In our study, AK prevalence was 0.01% for patients between 30 and 39 years of age, 0.45% for patients between 40 and 49 years of age, 1.77% for patients between 50 and 59 years of age, 4.61% for patients between 60 and 69 years of age, 9.38% for patients between 70 and 79 years of age, and 14.57% for patients ≥80 years. AK prevalence was 2.50% for the entire study group. In our study, as in previous studies, the incidence of AK increased with age in both sexes, and the incidence of AK was higher in males than in females.[4,7–10] Although the age and sex distributions of AK in our study were consistent with the rates reported in previous studies, and although our overall prevalence rate was similar to that of Schaefer et al[17] from Germany, it was lower than that of the studies reported from Switzerland, Austria, England, and America.[4,13,16] In addition to the methodological differences in the literature, we believe that these differences are related to the differing distributions of individual and environmental risk factors associated with AK development. The role of UV exposure in the development of SCC and AK has been discussed, investigated, and extensively confirmed.[4] Spending >2 weeks each year in the sun has been reported as a risk factor for AK development.[18] Other studies have also identified a close relationship between AK prevalence and localization in body regions frequently exposed to UV.[11] Dziunycz et al,[4] Eder et al,[11] Criscione et al,[19] and Hensen et al[9] reported that head regions receiving direct sunlight are the most common sites. In our study, in accordance with the literature, we also identified the head region as the most common site of AK. In addition, we identified a correlation between sun exposure and number of lesions, as well as a correlation between sun exposure and AK involvement in multiple regions. These findings support the role of UV exposure in AK development.
In previous studies, skin type has been reported as a risk factor for AK development, and AK development has been found to be more common in patients with skin types 1 and 2.[17,20] In our study, skin types 2 and 3 were the most common skin types among AK patients. There was also a correlation between the number of lesions and skin type. There were <5 lesions in all male patients with skin type 1. Skin type, UV exposure, advanced age, and male sex were determined as risk factors associated with AK development in previous studies.[11,21] We detected that age, UV exposure, and skin type were associated with a high AK load (P < .05).
The conversion rates of AKs to malignancy have been reported to be between 0.25% and 20% in different studies. The rate of conversion to malignancy in cases with >1 lesion was between 0.1% and 10%.[22,23] In our study, 17.67% of patients with AK had a history of nonmelanotic skin cancer. When these tumors were typed, in contrast to the findings of previous studies, it was observed that an association with BCC (10.62%) was the most common, and an association with SCC (7.05%) was the second most common.[22,23]
There are certain limitations to our study. The diagnoses of AK were not confirmed histopathologically. However, the clinical recognition of AK by dermatologists has been shown to have high diagnostic accuracy.[9,11] In addition, this study is retrospective and was limited to patients visiting dermatology offices; therefore, the population examined may not reflect the general population.
5. Conclusion
Given the excessive exposure to sunlight caused by the geographical location of our country, and given the tendency of AKs to convert to malignancy, it is important to identify the populations at high risk of AK development and to identify high-risk anatomical regions to form the basis of effective screening programs to support public health.
Author contributions
Conceptualization: mahizer yaldiz.
Data curation: mahizer yaldiz.
Investigation: mahizer yaldiz.
Methodology: mahizer yaldiz.
Resources: mahizer yaldiz.
Supervision: mahizer yaldiz.
Writing – original draft: mahizer yaldiz.
Writing – review & editing: mahizer yaldiz.
Footnotes
Abbreviations: AK = actinic keratosis, BCC = basal cell carcinoma, SCC = squamous cell carcinoma.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (name of institute/committee) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
For this type of study formal consent is not required. This article does not contain any studies with human participants performed by any of the authors.
The authors report no conflicts of interest.
References
- [1].Sacar H, Sacar T. Actinic Keratosis. Anatol J Clin Investig 2009;3:198–202. [Google Scholar]
- [2].Strickland PT, Vitasa BC, West SK, et al. Quantitative carcinogenesis in man: solar ultraviolet B dose dependence of skin cancer in Maryland watermen. J Natl Cancer Inst 1989;81:1910–3. [DOI] [PubMed] [Google Scholar]
- [3].Dziunycz PJ, Schuller E, Hofbauer GFL. Prevalence of actinic keratosis in patients attending general practitioners in Switzerland. Dermatology 2018;20:1–6. [DOI] [PubMed] [Google Scholar]
- [4].Trakatelli M, Barkitzi K, Apap C, et al. EPIDERM Group. Skin cancer risk in outdoor workers: a European multicenter case-control study. J Eur Acad Dermatol Venereol 2016;30:5–11. [DOI] [PubMed] [Google Scholar]
- [5].Werner RN, Sammain A, Erdmann R, et al. The natural history of actinic keratosis: a systematic review. Br J Dermatol 2013;169:502–18. [DOI] [PubMed] [Google Scholar]
- [6].Frost CA, Green AC, Williams GM. The prevalence and determinants of solar keratoses at a subtropical latitude (Queensland, Australia). Br J Dermatol 1998;139:1033–9. [DOI] [PubMed] [Google Scholar]
- [7].Green A, Battistutta D. Incidence and determinants of skin cancer in a high risk Australian population. Int J Cancer 1990;46:356–61. [DOI] [PubMed] [Google Scholar]
- [8].Trakatelli M, Ulrich C, del Marmol V, et al. Epidemiology of non melanoma skin cancer (NMSC) in Europe: Accurate and comparable data are needed for effective public health monitoring and interventions. Br J Dermatol 2007;156:1–7. [DOI] [PubMed] [Google Scholar]
- [9].Hensen P, Müller ML, Haschemi R, et al. Predisposing factors of actinic keratosis in a North-West German population. Eur J Dermatol 2009;19:345–54. [DOI] [PubMed] [Google Scholar]
- [10].Green AC. Epidemiology of actinic keratoses. Curr Probl Dermatol 2015;46:1–7. [DOI] [PubMed] [Google Scholar]
- [11].Eder J, Prillinger K, Korn A, et al. Prevalence of actinic keratosis among dermatology outpatients in Austria. Br J Dermatol 2014;171:1415–21. [DOI] [PubMed] [Google Scholar]
- [12].Memon AA, Tomenson JA, Bothwell J, et al. Prevalence of solar damage and actinic keratosis in a Merseyside population. Br J Dermatol 2000;142:1154–9. [DOI] [PubMed] [Google Scholar]
- [13].Naldi L, Chatenoud L, Piccitto R, et al. Prevalence of actinic keratoses and associated factors in a representative sample of the Italian adult population: results from the Prevalence of Actinic Keratoses Italian Study, 2003-2004. Arch Dermatol 2006;142:722–6. [DOI] [PubMed] [Google Scholar]
- [14].Harvey I, Frankel S, Marks R, et al. Non-melanoma skin cancer and solar keratoses II analytical results of the South Wales Skin Cancer Study. Br J Cancer 1996;74:1308–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Flohil SC, van der Leest RJ, Dowlatshahi EA, et al. Prevalence of actinic keratosis and its risk factors in the general population: the Rotterdam Study. J Invest Dermatol 2013;133:1971–8. [DOI] [PubMed] [Google Scholar]
- [16].Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer 2009;115:2523–30. [DOI] [PubMed] [Google Scholar]
- [17].Youl PH, Janda M, Aitken JF, et al. Body site distribution of skin cancer, premalignant and common benign pigmented lesions excised in general practice. Br J Dermatol 2011;165:35–43. [DOI] [PubMed] [Google Scholar]
- [18].Pavone PS, Lovati S, Scarcella G, et al. Efficacy of different photoprotection strategies in preventing actinic keratosis new lesions after photodynamic therapy. The ATHENA study: a two-center, randomized, prospective, assessor-blinded pragmatic trial. Curr Med Res Opin 2018;8:1–1. [DOI] [PubMed] [Google Scholar]
- [19].Wheller LH, Soyer P. Clinical features of actinic keratoses and early squamous cell carcinoma. Curr Probl Dermatol (Basel, Karger) 2015;46:58–63. [DOI] [PubMed] [Google Scholar]
- [20].Lucas R, McMichael T, Smith W, et al. Solar ultraviolet radiation: global burden of disease from solar ultraviolet radiation. Environmental Burden of Disease Series No. 13. 2006;Geneva (Switzerland): World Health Organization, 187. [Google Scholar]
- [21].Berman B, Cockerell CJ. Pathobiology of actinic keratosis: ultraviolet-dependent keratinocyte proliferation. J Am Acad Dermatol 2013;68:10–9. [DOI] [PubMed] [Google Scholar]
- [22].Traianou A, Ulrich M, Apalla Z, et al. Risk factors for actinic keratosis in eight European centres: a case-control study. Br J Dermatol 2012;167:36–42. [DOI] [PubMed] [Google Scholar]
- [23].Schaefer I, Augustin M, Spehr C, et al. Prevalence and risk factors of actinic keratoses in Germany analysis of multisource data. J Eur Acad Dermatol Venereol 2014;28:309–13. [DOI] [PubMed] [Google Scholar]


