Supplemental Digital Content is available in the text
Keywords: end-stage renal disease, risk, systemic lupus erythematosus
Abstract
Systemic lupus erythematosus (SLE) is known to be one of the leading causes of end-stage renal disease (ESRD). The aim of this study was to estimate the incidence rate of ESRD and the risk for progression to ESRD in SLE patients compared to the general population.
A total of 21,253 SLE patients were extracted from the Korean National Health Insurance Service database between 2008 and 2013. Age-and sex-matched controls (n = 106,265) were randomly sampled in a 5:1 ratio from non-SLE individuals. Both cohorts were followed up for development of ESRD until 2015.
During the median 7.53 years of follow-up, 533 (2.51%) cases of ESRD were newly developed in SLE patients and 145 (0.14%) cases in matched controls (incidence rate: 4.075 and 0.219 per 1000 person-year, respectively). SLE patients were at higher risk for ESRD development compared to matched controls (hazard ratio [HR], 9.84; 95% confidence interval [CI] 8.10–11.96) after multivariate adjustment. In subgroup analysis, the risk for ESRD was higher in male (HR, 7.76; 95% CI 5.07–11.90) and female patients with SLE (HR, 10.48; 95% CI 8.41–13.07) than in matched controls. When analyzed by age group, the younger the age, the higher the risk of ESRD versus non-SLE matched controls; this result was also significant after adjustment. In subgroup analysis according to comorbidities, the SLE group had a significantly higher risk of ESRD than the non-SLE group in almost all subgroups.
SLE was associated with an increased incidence of ESRD.
1. Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that is typically characterized by the formation of autoantibodies such as antinuclear antibodies and anti-double-stranded DNA antibodies. SLE has diverse clinical presentations involving multiple organs including the kidney. Renal involvement is one of the most frequent and serious complications of SLE, and the presentation varies from subclinical laboratory abnormalities to overt nephritis or nephrotic syndrome. Further, SLE is one of the major causes of chronic kidney disease (CKD) and end-stage renal disease (ESRD). It is known that renal involvement occurs in approximately 60% of SLE patients,[1] and 10% to 20% of them progress to ESRD.[2] CKD and ESRD caused by lupus nephritis are major causes of mortality in SLE patients.[1] Although there have been recent advances in immunosuppressive treatment, progression to ESRD and mortality has not declined in the last decades.[1,3]
The prevalence and incidence of SLE are known to vary by race, and renal involvement tends to be more common in Asian patients.[4,5] Therefore, it is very important to evaluate the characteristics, risk factors, and prognosis of diseases that are specific to a race or country and to apply them in therapy. The aim of this nationwide cohort study was to investigate the incidence rate of ESRD and risk factors for progression to ESRD in Korean SLE patients compared to the general population.
2. Materials and methods
2.1. Data source and study population
The Korean National Health Insurance System (NHIS), which includes an eligibility database (age, sex, socioeconomic variables, type of eligibility, income level, etc), a medical treatment database (based on the accounts that were submitted by medical service providers for their medical expenses), a health examination database (results of general health examinations and questionnaires on lifestyle and behavior), and a medical care institution database (types of medical care institutions, location, equipment, and number of physicians), comprises a complete set of health information pertaining to 50 million Koreans.[6–8] In Korea, the NHIS is the only insurer, is managed by the government, and is subscribed to by approximately 97% of the Korean population. The remaining 3% of the population is covered by the Medical Aid program. In addition, the total claim rate for medical expenses is 100%. Therefore, it can be said that there are few people missing in the NHIS cohort among the Korean population.
Diagnosis statements were defined by the International Classification of Diseases, tenth revision (ICD-10). All patients in whom SLE was diagnosed (International Classification of Diseases, 10th revision [ICD-10] codes M32 and special medical aid code V136) between 2008 and 2013 were extracted from the database. New patients for whom the SLE code had not been previously entered were included. Of these, patients younger than 20 years or those with a history of ESRD during a washout period from 2002 to 2007 were excluded. For the non-SLE control cohort, age-and sex-matched controls (n = 106,265) were randomly sampled from non-SLE individuals in 2008∼2013 at a 5:1 ratio of controls to cases. Randomization was done using an algorithm within the SAS version 9.3 program (SAS Institute, Cary, NC).
2.2. Definitions of comorbidities
Income level was dichotomized at the lowest 20%. The presence of diabetes mellitus was defined according to the following criteria: at least 1 claim per year under ICD-10 codes E11–14 and at least 1 claim per year for the prescription of antidiabetic medication. The presence of hypertension was defined according to the presence of at least 1 claim per year under ICD-10 codes I10∼I13 and I15 and at least one claim per year for the prescription of an antihypertensive agent. The presence of dyslipidemia was defined according to the presence of at least 1 claim per year under ICD-10 code E78 and at least 1 claim per year for the prescription of a lipid-lowering agent. The presence of myocardial infarction (MI) was defined as at least one claim under ICD-10 codes I21, I22, Congestive heart failure (CHF) as at least 1 claim under ICD-10 code I50, and chronic obstructive pulmonary disease (COPD) as at least 1 claim code J41∼J44 during the 3 years before the entry year. The presence of stroke was defined as at least 1 claim under ICD-10 codes I63 and I64, and prescription of brain computed tomography or magnetic resonance imaging during the 3 years before the entry year. Blood samples for the measurement of serum creatinine were drawn from patients after an overnight fast. The estimated glomerular filtration rate (eGFR) was calculated using the modification of diet in renal disease study formula, and CKD was defined as eGFR <60 mL/min/1.73 m2.
2.3. Study outcomes and follow-up
The study population was followed from baseline to the date of ESRD diagnosis or until December 31, 2015, whichever came first. The primary endpoint was incident ESRD, which was defined using the combination of ICD-10 codes (N18 and N19) and special medical aid codes for renal replacement therapy (V001 for hemodialysis, V003 for peritoneal dialysis, and V005 for kidney transplantation). All medical care expenses for dialysis are reimbursed using the Korean Health Insurance Review and Assessment Service database. These patients are also registered as special medical aid beneficiaries. Therefore, we were able to identify every ESRD patient in the whole of the South Korean population and to analyze the data for all ESRD patients who had started dialysis. Codes for treatment or medical expense claims included R3280 for kidney transplantation, O7011-O7020 or V001 for hemodialysis, and O7071-O7075 or V003 for peritoneal dialysis. We excluded individuals with previous ESRD who had a transplant or dialysis code on the same date as an acute renal failure code. Subjects on continuous renal replacement therapy or acute peritoneal dialysis were also excluded.
2.4. Statistical analysis
The participants’ baseline characteristics are presented as means ± standard deviation or n (%). The incidence rates of primary outcomes were calculated by dividing the number of incident cases by the total follow-up period. The incidence rates of the primary outcomes are presented as per 1000 person-years. The disease-free probability of a primary outcome according to the presence of SLE was calculated using Kaplan-Meier curves, and the log-rank test was used to analyze differences between groups. Hazard ratios (HRs) and 95% confidence interval (CI) values for ESRD among groups were analyzed using Cox proportional hazard models. Multivariable-adjusted proportional hazard models were applied as follows: model 1 was not adjusted; model 2 was adjusted for age and sex; model 3 was further adjusted for lower income status, diabetes mellitus, hypertension, dyslipidemia, MI, stroke, and CHF. In model 2 and 3, we performed an adjustment for age and sex in consideration of their effect over time on the survival analysis and its relevance to other factors. Statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC), and a P value <.05 was considered to indicate significance.
2.5. Ethics statement
This study was approved by the Institutional Review Board of the Chonnam National University Hospital of Korea (CNUH-EXP-2018–026) and Institutional Review Board of the Republic of Korea National Institute for Bioethics Policy (NHIS-2018–1–128). Anonymized and deidentified information were used for the analyses, and thus, the need for informed consent was waived.
3. Results
3.1. Baseline characteristics of the study population
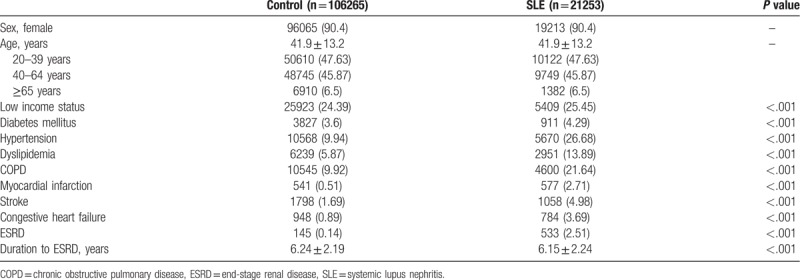
The characteristics of participants classified by the presence of SLE are described in Table 1. A total of 21,253 patients were recruited for the SLE cohort: 19,213 (90.4%) were women, the mean age was 41.9 ± 13.2 years, and 106,265 age and sex-matched non-SLE cohort individuals were selected as the control. Compared to the control group, the SLE group had lower income distributions and a significantly higher prevalence of comorbidities related to ESRD, such as diabetes mellitus (4.29%), hypertension (26.68%), dyslipidemia (13.89%), COPD (21.64%), MI (2.71%), stroke (4.98%), and CHF (3.69%).
Table 1.
Baseline characteristics of SLE patients and matched controls.
3.2. Incidence risk of ESRD in SLE patients
During a median 7.53 years of follow-up after the diagnosis of SLE, 533 (2.51%) participants developed ESRD, while 145 (0.14%) non-SLE participants developed ESRD (Table 1). In addition, the mean duration of progression to ESRD was significantly shorter in the SLE group than in the non-SLE group (6.15 ± 2.24 years and 6.24 ± 2.19 years, respectively, P <.001) (Table 1). The incidence probability of ESRD according to the presence of SLE is shown in Figure 1. The SLE group had a significantly higher incidence of ESRD than the non-SLE group (P of log-rank test <.001).
Figure 1.
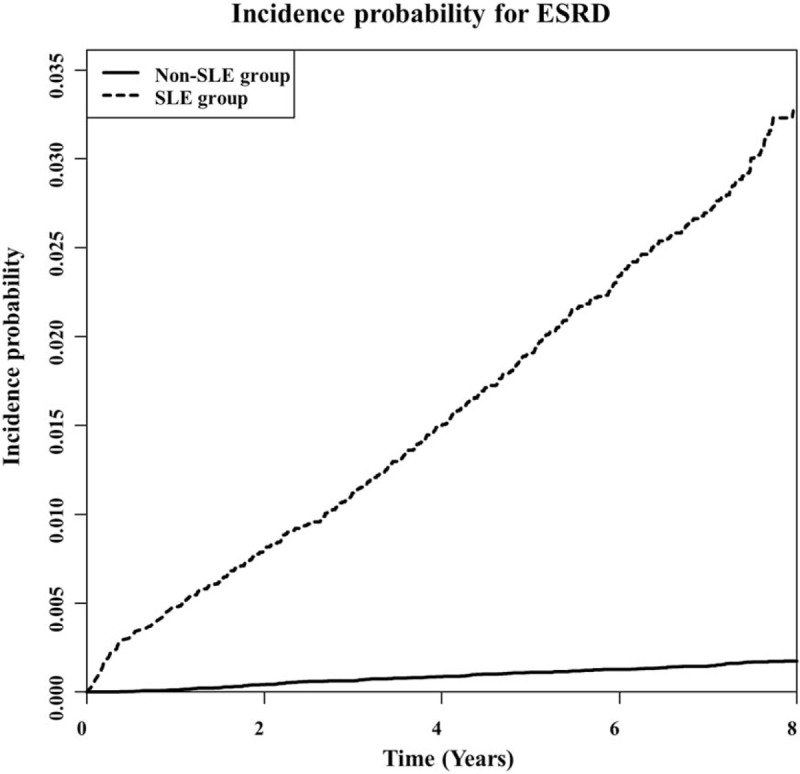
Kaplan–Meier curve for 8-year crude cumulative incidence of ESRD between SLE and non-SLE patients. (P of log-rank <.001). ESRD = end-stage renal disease, SLE = systemic lupus nephritis.
3.3. Subgroup analysis of risk of ESRD in SLE patients
The incidence rate of ESRD was higher in the SLE group (4.075/1000 person-years) than in the non-SLE group (0.219/1000 person-years) (Table 2 ). The crude hazard ratio (HR) for ESRD was higher for the SLE group (HR 18.64; 95%, confidence interval [CI], 15.56–22.47) than for the non-SLE group (Table 2 , Model 1). After multivariate adjustment for age, sex, income, diabetes mellitus, hypertension, dyslipidemia, MI, stroke, and CHF, there was still a significant difference in ESRD risk between the 2 groups (HR, 9.84; 95% CI, 8.10–11.97) (Table 2 , Model 3). In the subgroup analysis, male (HR, 7.76; 95% CI, 5.07–11.90) and female patients (HR, 10.479; 95% CI, 8.405–13.066) with SLE had a higher risk of ESRD than the non-SLE matched controls. When analyzed by age group, the younger the age, the higher the risk of ESRD versus non-SLE matched control, which was also significant after adjustment. The risk for ESRD was higher in 20 to 39-year-old patients with SLE (HR, 22.41; 95% CI 14.20–35.36), 40–64-year-old patients with SLE (HR, 8.46; 95% CI 6.42–11.13), and ≥65-year-old patients with SLE (HR, 5.01; 95% CI 3.34–7.53) than in matched controls. In subgroup analysis according to income status and comorbidities, the SLE group had a significantly higher risk of ESRD than the non-SLE group in almost all subgroups (the only exception was the MI subgroup).
Table 2.
Association of SLE and incident ESRD.
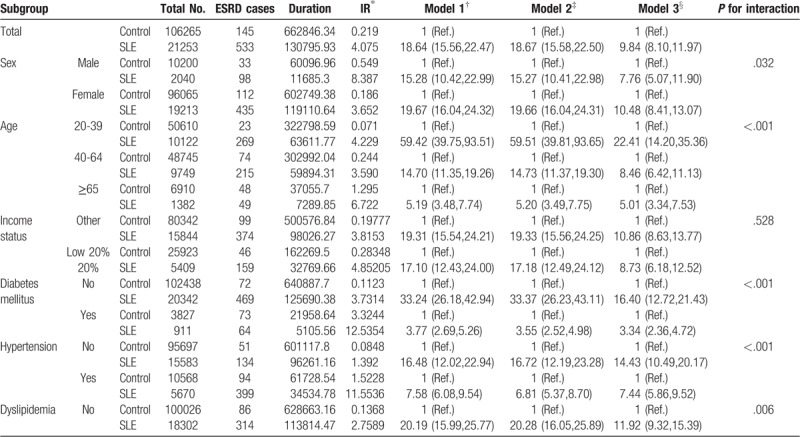
Table 2 (Continued).
Association of SLE and incident ESRD.
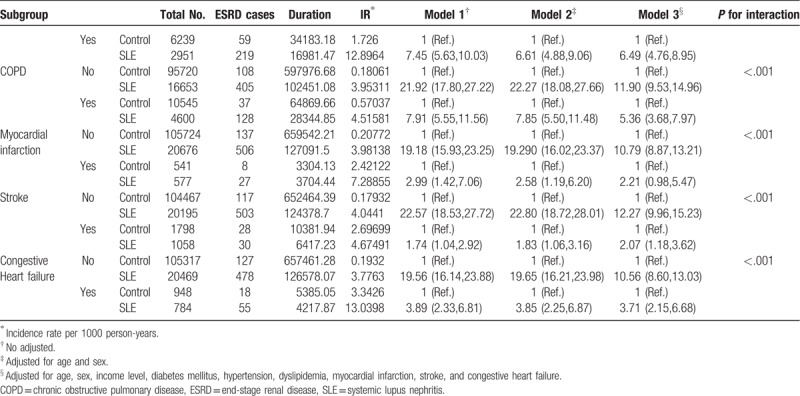
We further performed adjustment for CKD status and subgroup analysis according to the presence of CKD. Only patients who had a serum creatinine value from a health examination performed within 1 year after diagnosis of SLE were analyzed. Even after full adjustment for comorbidities, the population with SLE had a higher risk for ESRD than the matched controls (Fig. 2 and supplementary Table 1). The risk of incident ESRD was higher in both the non-CKD and CKD subgroups than in the population without SLE.
Figure 2.
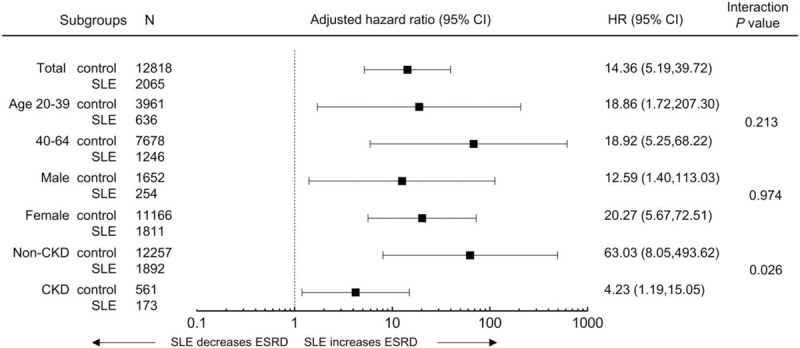
Analysis of end-stage renal disease in subgroups of patients with SLE. HRs and 95% CIs of end-stage renal disease in the SLE group vs control in subgroups. Adjusted for age, sex, income, diabetes mellitus, hypertension, dyslipidemia, myocardial infarction, stroke, congestive heart failure, and CKD. CI = confidence interval, CKD = chronic kidney disease, HR = hazard ratios.
4. Discussion
ESRD is not an uncommon complication in SLE. Despite active chemotherapy in patients with lupus nephritis, a significant proportion of SLE patients progress to ESRD.[9,10] In this population-based cohort study, we investigated the incidence rate of ESRD and risk factors for progression to ESRD in Korean SLE patients compared to the general population. Our results showed that SLE was associated with a higher risk of ESRD development and shorter duration of progression to ESRD during the follow-up period. In subgroup analyses by age or sex, SLE was still a risk factor for ESRD, even after the adjustment for comorbidities. The present study is the first attempt to investigate the incidence and risk factors for ESRD in SLE patients from a nationwide cohort in Korea.
Several previous studies have analyzed the incidence and risk factors of ESRD in patients with SLE. Croca et al[1] observed the incidence of ESRD in a British lupus nephritis cohort for 30 years, and 30 out of 154 lupus nephritis patients (19.5%) developed ESRD, with an average time to progression to ESRD of 7.5 years. The rate of progression to ESRD 5 years after the diagnosis of lupus nephritis was not statistically significant among the first to third decades of life, indicating no difference in the risk of developing ESRD over time.[1] Studies on the occurrence of ESRD in SLE patients using United States Renal Data System (USRDS) data have been conducted since the 1980s.[3,11–13] Recently, Sexton et al[11] reported the standardized incidence ratio of ESRD in lupus nephritis patients of approximately 1.05 cases per million patient-years during the biennium period from 2009 to 2010 using the USRDS data.
It is well known from previous studies that the epidemiology, course, disease pattern and activity, complications, and prognosis of SLE are largely different depending on race or ethnicity.[4,14–18] Asian patients have been reported to have higher rates of lupus nephritis and more active glomerulonephritis compared to Caucasian patients.[19] Renal outcome and the level of immunosuppressant use in Asians were comparable to African-American Blacks in some studies. Joo et al[20] reported the clinical features and organ damage in 996 Korean patients with SLE compared with other Asian SLE patients. Compared to other Asian cohorts, disease activity was lower and organ damage was less in Korean SLE patients. During the follow-up period of 10 years, lupus nephritis increased over time, and eventually, 50% of the patients developed lupus nephritis and 3% of patients required dialysis. However, considering that most of the above previous studies were cohort studies, direct comparisons with our results are not appropriate. Although nationwide population-based studies of SLE patients have been conducted in Korea, there have been no studies focused on ESRD.[21,22] Based on the largest population ever studied, we analyzed the risk of incident ESRD in a Korean population with SLE. SLE was associated with a significant increase in the risk of ESRD even after adjustment for comorbidities. In the subgroup analysis, SLE increased the risk of ESRD regardless of the presence of various diseases. Especially in the subgroup analysis according to CKD, SLE significantly increased the incidence of ESRD not only in patients without baseline CKD, but also in patients with CKD.
A nationwide population-based study similar to ours was conducted in Taiwan.[23] Taiwan's national health insurance system is similar to that of Korea. Yu et al[23] used Taiwan's Longitudinal Health Insurance Database to analyze the risk of ESRD in SLE patients. The incidence rates of ESRD were 612.8 and 29.3 cases per 100,000 patient-years in the SLE and non-SLE groups, respectively. The incidence of ESRD occurred more frequently in male patients than in female patients, and the HR for ESRD was 18.2 times higher in the SLE group than in the non-SLE group. Our results showed that the incidence rate of ESRD in Korean SLE patients (4.075/1000 person-year) was lower than that of Taiwanese patients. This is consistent with the findings reported in previous studies.[20]
This study was the first nationwide population-based study in Korea for the incidence and risk factors of ESRD in SLE patients. Due to the special nature of the Korean health insurance system, in which the insured are covered by a single compulsory insurance with a 100% claim rate, the number of patients who are missing is small, because information on all SLE and ESRD patients being treated in the hospital is collected by the insurer. However, the present study has some limitations. First, patients not treated in the hospital were not included in the study because they are not recorded in the claim data. Therefore, the prevalence or incidence of SLE may be underestimated rather than actual. Second, claim data does not include laboratory results and detailed history including information on serum creatinine and proteinuria of patients that may affect the outcome of the study. To overcome this, history of major diseases such as hypertension, diabetes, and cardiovascular disease were also analyzed by ICD-10 code of the claim data. Third, lupus nephritis could not be validated because the claim data do not include the results of renal biopsy or the presence of proteinuria.
In summary, this study showed a high incidence rate of ESRD in SLE patients. SLE patients had a 10-fold greater risk of ESRD when compared to non-SLE patients. Despite some limitations, this is the first study of the incidence and the risk of ESRD in Korean patients with SLE, which was performed using Korean National Health Insurance Service database. The results of this study would be helpful in establishing a national health policy for the management of SLE patients.
Author contributions
Conceptualization: Hong Sang Choi, Kyung-Do Han, Soo Wan Kim.
Data curation: Kyung-Do Han, Jin-Hyung Jung.
Formal analysis: Hong Sang Choi, Kyung-Do Han, Jin-Hyung Jung, Chang Seong Kim, Eun Hui Bae, Seong Kwon Ma, Soo Wan Kim.
Investigation: Chang Seong Kim.
Methodology: Kyung-Do Han, Chang Seong Kim.
Resources: Kyung-Do Han.
Software: Kyung-Do Han.
Supervision: Eun Hui Bae, Seong Kwon Ma, Soo Wan Kim.
Validation: Kyung-Do Han, Jin-Hyung Jung, Eun Hui Bae, Seong Kwon Ma.
Visualization: Chang Seong Kim, Eun Hui Bae, Soo Wan Kim.
Writing – original draft: Hong Sang Choi.
Writing – review & editing: Chang Seong Kim, Eun Hui Bae, Seong Kwon Ma, Soo Wan Kim.
Supplementary Material
Footnotes
Abbreviations: CHF = congestive heart failure, CI = confidence interval, CKD = chronic kidney disease, COPD = chronic obstructive pulmonary disease, eGFR = estimated glomerular filtration rate, ESRD = end-stage renal disease, HR = hazard ratio, ICD-10 = International Classification of Diseases, 10th revision, MI = myocardial infarction, NHIS = National Health Insurance System, SLE = systemic lupus erythematosus, USRDS = United States Renal Data System.
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) grant funded by the Ministry of Education (NRF-2016R1D1A1B03931229), by a grant (BCRI18024) of Chonnam National University Hospital Biomedical Research Institute, and by the Bio & Medical Technology Development Program of the National Research Foundation of Korea (NRF) funded by the Korean government, MSIT (2017M3A9E8023001 and 2017M3A9E8023016).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Croca SC, Rodrigues T, Isenberg DA. Assessment of a lupus nephritis cohort over a 30-year period. Rheumatology (Oxford) 2011;50:1424–30. [DOI] [PubMed] [Google Scholar]
- [2].Maroz N, Segal MS. Lupus nephritis and end-stage kidney disease. Am J Med Sci 2013;346:319–23. [DOI] [PubMed] [Google Scholar]
- [3].Costenbader KH, Desai A, Alarcon GS, et al. Trends in the incidence, demographics, and outcomes of end-stage renal disease due to lupus nephritis in the US from 1995 to 2006. Arthritis Rheum 2011;63:1681–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Crosslin KL, Wiginton KL. The impact of race and ethnicity on disease severity in systemic lupus erythematosus. Ethn Dis 2009;19:301–7. [PubMed] [Google Scholar]
- [5].Jakes RW, Bae SC, Louthrenoo W, et al. Systematic review of the epidemiology of systemic lupus erythematosus in the Asia-Pacific region: prevalence, incidence, clinical features, and mortality. Arthritis Care Res (Hoboken) 2012;64:159–68. [DOI] [PubMed] [Google Scholar]
- [6].Song SO, Jung CH, Song YD, et al. Background and data configuration process of a nationwide population-based study using the korean national health insurance system. Diabetes Metab J 2014;38:395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lee SR, Choi EK, Rhee TM, et al. Evaluation of the association between diabetic retinopathy and the incidence of atrial fibrillation: a nationwide population-based study. Int J Cardiol 2016;223:953–7. [DOI] [PubMed] [Google Scholar]
- [8].Yang HK, Han K, Kwon HS, et al. Obesity, metabolic health, and mortality in adults: a nationwide population-based study in Korea. Sci Rep 2016;6:30329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Faurschou M, Dreyer L, Kamper AL, et al. Long-term mortality and renal outcome in a cohort of 100 patients with lupus nephritis. Arthritis Care Res (Hoboken) 2010;62:873–80. [DOI] [PubMed] [Google Scholar]
- [10].Yap DY, Tang CS, Ma MK, et al. Survival analysis and causes of mortality in patients with lupus nephritis. Nephrol Dial Transplant 2012;27:3248–54. [DOI] [PubMed] [Google Scholar]
- [11].Sexton DJ, Reule S, Solid C, et al. ESRD from lupus nephritis in the United States, 1995-2010. Clin J Am Soc Nephrol 2015;10:251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ward MM. Changes in the incidence of end-stage renal disease due to lupus nephritis, 1982-1995. Arch Intern Med 2000;160:3136–40. [DOI] [PubMed] [Google Scholar]
- [13].Ward MM. Changes in the incidence of endstage renal disease due to lupus nephritis in the United States, 1996-2004. J Rheumatol 2009;36:63–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Barbhaiya M, Feldman CH, Guan H, et al. Race/ethnicity and cardiovascular events among patients with systemic lupus erythematosus. Arthritis Rheumatol 2017;69:1823–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Falasinnu T, Chaichian Y, Bass MB, et al. The representation of gender and race/ethnic groups in randomized clinical trials of individuals with systemic lupus erythematosus. Curr Rheumatol Rep 2018;20:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ghaussy NO, Sibbitt W, Jr, Bankhurst AD, et al. The effect of race on disease activity in systemic lupus erythematosus. J Rheumatol 2004;31:915–9. [PubMed] [Google Scholar]
- [17].Gonzalez LA, Toloza SM, Alarcon GS. Impact of race and ethnicity in the course and outcome of systemic lupus erythematosus. Rheum Dis Clin North Am 2014;40:433–54. [DOI] [PubMed] [Google Scholar]
- [18].Isenberg D, Appel GB, Contreras G, et al. Influence of race/ethnicity on response to lupus nephritis treatment: the ALMS study. Rheumatology (Oxford) 2010;49:128–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mok MY, Li WL. Do Asian patients have worse lupus. Lupus 2010;19:1384–90. [DOI] [PubMed] [Google Scholar]
- [20].Joo YB, Bae SC. Assessment of clinical manifestations, disease activity and organ damage in 996 Korean patients with systemic lupus erythematosus: comparison with other Asian populations. Int J Rheum Dis 2015;18:117–28. [DOI] [PubMed] [Google Scholar]
- [21].Ju JH, Yoon SH, Kang KY, et al. Prevalence of systemic lupus erythematosus in South Korea: an administrative database study. J Epidemiol 2014;24:295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shim JS, Sung YK, Joo YB, et al. Prevalence and incidence of systemic lupus erythematosus in South Korea. Rheumatol Int 2014;34:909–17. [DOI] [PubMed] [Google Scholar]
- [23].Yu KH, Kuo CF, Chou IJ, et al. Risk of end-stage renal disease in systemic lupus erythematosus patients: a nationwide population-based study. Int J Rheum Dis 2016;19:1175–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


