Abstract
Obstructive sleep apnea (OSA) adversely affects neurological recovery. This study aimed to determine the impact of continuous positive airway pressure (CPAP) and/or rehabilitation in basal ganglia stroke patients with OSA.
A prospective controlled trial was conducted in 2015–2018. The subjects received routine rehabilitation training for up to 2 years and were assigned to the intervention and control groups treated with CPAP or without, respectively. Then, treatment effects on sleep parameters, motor function, stroke severity, daily life activities, cognitive function, and psychological states were assessed at different time points.
At 6 months, the CPAP group showed significantly lower mean apnea-hypopnea index (AHI), percentage of time with SpO2 at <90% (TS90%), micro-arousal index, and percentages of time in non-rapid eye movement (non-REM) stages 1–2 and REM stage in total sleeping time compared with the control group, and significantly higher mean minimum of peripheral oxygen saturation (L-SaO2%) and percentage of time in stage 3 (P < .001) sleep. The CPAP group showed significant improvements in average the National Institutes of Health Stroke Scale (NIHSS), Fugl-Meyer assessment scale (FMA), Barthel index (BI), Minimental state examination (MMSE), Hamilton anxiety scale (HAMA) and Hamilton depression rating scale for depression (HRSD) scores at different times versus the control group, respectively (P < .05). However, no difference in body mass index (BMI) management was observed (P > .05). Repeated-measures ANOVA revealed significant interactions between the two groups for change in FMA, MMSE, BI, HAMA, and HRSD scores from admission to 24 months (P < .001), but no significant was found for BMI (P = .582).
Basal ganglia stroke patients with OSA tend to have significantly greater sleeping, neurological and functional recovery after CPAP, and rehabilitation over 2 years.
Keywords: continuous positive airway pressure, obstructive sleep apnea, rehabilitation, stroke
1. Introduction
Stroke is the second most significant cause of death, and the third leading cause of disability-adjusted life-years worldwide.[1] Every year, there are more than 10 million major strokes around the world,[2] due to the negative impact of population aging and rapid urbanization. China, the world's most populous country, however, is likely to bear a major burden.[1,3] Some mechanisms have been discovered, for example, the levels of factor VII (FVII), factor VIIa-antithrombin complexes (FVIIa-AT), and total tissue factor (TF) are decreased, while tissue factor-bearing microparticles (MPs-TF) are elevated in patients with ischemic stroke.[4] However, the role of inflammation in cerebrovascular disease is complex, activation of renin-angiotensin-aldosterone system (RAAS), through increase in the production of angiotensin II (Ang II), is closely related to local vascular inflammation.[5] A study confirm that stroke patients exhibited significantly higher plasma levels of cytokines, selectins, adhesion molecules and plasminogen activator inhibitor-1 (PAI-1), and diabetic patients with lacunar strokes exhibited a minor grade of immunoinflammatory activation of the acute phase at 24–72 h and 7–10 days after stroke onset.[6] Therefore, predictors of good prognosis, in terms of in-hospital mortality and cognitive and functional performance at discharge, included pretreatment with ACE-inhibitors, calcium channel blockers and anti-platelets.[7]
The fundamental purpose of rehabilitation treatment for stroke is to prevent complications, reduce obstacles and improve body functions to the greatest extent, while improving daily life activities.[8] Obstructive sleep apnea (OSA) is the most common sleep-related breathing disorder. It refers to the condition of sleep-disordered breathing, whereby cessation of airflow occurs due to upper airway collapse. This results in a disruption of normal sleep physiology, and manifests as snoring, excessive daytime sleepiness, morning headache, sexual dysfunction, and cognitive and mood disorders, and could probably lead to stroke and other systemic ailments.[9,10] A review on the epidemiology of sleep apnea by Franklin KA, with a refined and uniform methodology, revealed mean prevalence rates of 22% and 17% in men and women, respectively,[11] which was also shown in another recent study confirming moderate to severe OSA in up to 20% of the middle-aged population.[12] As a major public health concern, OSA is an independent risk factor for stroke and death.[13,14] OSA causes acute physiological changes, including alveolar hypoventilation and pulmonary artery vasoconstriction; it also promotes chronic vascular disease secondary to increased platelet adhesiveness, endothelial dysfunction, and accelerated atherosclerosis.[15] In addition, OSA is responsible for sleep fragmentation, which potentially damages neurological and cognitive functions.[16] OSA is characterized by repetitive episodes of complete or partial obstruction of the upper airway, which can occur in any stage of sleep but is more frequently in non-rapid eye movement (non-REM) stages 1 and 2 and rapid eye movement (REM) sleep in comparison with non-REM stage 3.[17] These episodes often lead to reduced blood oxygen saturation and body movement that can cause brain arousal and sympathetic activation.[18]
OSA prevalence can rise to 72% in stroke patients.[19] Meanwhile, stroke patients with OSA show lower functional capacity and spent longer time for recovery compared with non-OSA stroke cases.[20,21] Treatment of OSA with continuous positive airway pressure (CPAP) reduces the incidence of stroke.[15] Studies revealed that CPAP reduces stroke recurrence and improves recovery with feasible initiation in stroke patients with OSA.[22]
Estimates suggest, however, that as many as 70–80% of patients with OSA are neither diagnosed nor treated.[23] Moreover, due to physical disability and relative inaccessibility of polysomnography (PSG) to detect OSA, stroke patients frequently remain undiagnosed and untreated. The American Heart Association/American Stroke Association secondary stroke prevention guidelines recommend that patients with ischemic stroke and TIA receive screening for sleep apnea and treatment because of data suggesting that post-cerebrovascular event outcomes are improved after treatment of sleep apnea.[24] However, these recommendations have not been adopted widely. Although CPAP is the mainstay therapy for OSA, compliance remains variable. Nasal disease and nasal parameters,[25] temperature and humidity,[26] and mask and interface[27] are important factors for CPAP therapy discontinuation and long-term adherence.
Thus, a correct diagnosis of OSA in stroke patients and subsequent rehabilitation has become prominently important. As a potentially modifiable risk factor for stroke, its treatment and prevention could represent an important strategy for OSA with stroke. To identify a method with which proper OSA therapy optimizes stroke rehabilitation, we recruited patients with basal ganglia stroke (ischemic and hemorrhagic) with OSA. This prospective study focused on the impact of CPAP and/or rehabilitation on acute stroke with OSA, and recovery from stroke in short and long terms. We aimed to assess the role of CPAP as an important factor affecting the outcome after stroke.
2. Design and methods
The present study was a prospective multicenter controlled trial performed at two medical centers, including the Department of Respiratory Medicine, the Second Affiliated Hospital of Suzhou University, and the Department of Respiratory Rehabilitation and Neurology Rehabilitation, The Fourth Rehabilitation Hospital of Shanghai, China. Briefly, stroke patients with OSA were recruited over a period of 42 months from January 2015 to June 2018 in this study. We specifically focused on basal ganglia stroke (ischemic and hemorrhagic) as it is more prevalent and to avoid bias. The subjects were assigned to the intervention and control groups, respectively, and long-term treated with or without CPAP (Dream Station Auto CPAP (CNX500S17), Respironics, Inc, and AirSense 10 AutoSet Plus, ResMed Limited). This study was approved by the Ethics Committee of Suzhou University and The Fourth Rehabilitation Hospital of Shanghai, and written informed consent was obtained from each participant (Trial Registration: ChiCTR1900020729).
3. Patient definition
Patients were enrolled in this study according to the following criteria:
-
1.
stroke confirmed by a neurologist,
-
2.
age between 36 and 80 years,
-
3.
admission within 4 weeks of stroke onset,
-
4.
ability to participate in the sleep study and neuropsychological assessment, and
-
5.
sufficiently fluency in lingual communication.
Exclusion criteria were:
-
1.
severe, unstable medical conditions, respiratory failure, or history of severe congestive heart failure;
-
2.
traumatic brain injury;
-
3.
severe aphasia, confusion, or severe psychiatric comorbidity;
-
4.
central sleep apnea;
-
5.
any previously diagnosed sleep diseases, including narcolepsy, periodic limb movement disorder and Parkinson's disease-related sleep disorders;
-
6.
treatment for previously diagnosed OSA;
-
7.
more than 4 weeks after stroke onset;
-
8.
previous upper airway surgery.
4. Measurements
4.1. Polysomnographic study
All stroke patients underwent one night (≥8 h) of polysomnographic (PSG) study at the Sleep laboratory of our department within 4 weeks of stroke onset. They underwent the PSG test without drinking alcoholic or caffeinated drinks for the last 2 days, under bedside supervision. Overnight sleep polysomnography (SOMNO check 2; Wanman; Germany) included electroencephalography (C3/A2, C4/A1, O1/A2, and O2/A1), electrooculography, submental electromyography, bilateral anterior tibialis electromyography, electrocardiography, nasal airflow measurement (sensed by both thermistor and pressure transducer), and monitoring of thoracoabdominal movements, oxygen saturation, snoring, and body position. Sleep stages and respiratory events were analyzed based on the Sleep Medicine Criteria (American Academy, 2007).[28] Apnea was defined as a reduction of airflow of ≥90% for at least 10 s, and hypopnea as a reduction of airflow of ≥50% for at least 10 s followed by oxygen desaturation of ≥3%. Apneas with thoracic motion, without thoracic motion, and with initial lack of motion followed by respiratory effort, were classified as obstructive, central, and mixed apneas, respectively. OSA was diagnosed when at least 50% of respiratory events were of the obstructive type. The apnea-hypopnea index (AHI) was defined as the mean number of apneas and hypopneas per hour in bed. Other OSA-associated respiratory parameters, including minimum of peripheral oxygen saturation (SpO2), mean of SpO2 and percentage of time with SpO2 at <90%, were recorded. Sleep architecture variables were also examined, including sleep efficiency, sleep stages, and the micro-arousal index. Sleep efficiency was defined as the percentage of sleeping duration divided by the total time spent in bed. The micro-arousal index was the number per hour of micro-arousals lasting 3–15 s. Patients with oxygen desaturation observed by polysomnography received one night of oximetry while using CPAP. CPAP pressure was adjusted by monitoring chest and abdomen movement, oronasal airflow and finger blood oxygen saturation, which gradually increased from 4 cmH2O (1 cmH2O = 0.098 kPa) to apnea disappearance and finger blood oxygen saturation (SaO2) >90%. And those with persistent desaturation despite auto-titrating CPAP were provided supplemental nocturnal oxygen via the CPAP machine. The average pressure of CPAP was (8 ± 4) cmH2O.
4.2. Obstructive sleep apnea treatment
CPAP adherence was classified as ’none’ if the patient refused to take a receipt from the CPAP machine or did not use it at all, ’poor’ with the number of nights of usage was ≤10% of all nights available, 'some’ with the nights used >10% of total nights available or cumulative hours of use <4 h per night times 70% of all nights available for use, and ’excellent’ if total cumulative hours of use were ≥4 h per night times 70% of all nights available for use.[29] The primary outcome related to OSA treatment was the proportion of treated patients with excellent CPAP adherence.
4.3. CPAP adherence support
Our approach to improving CPAP adherence consisted of early intensive education and support, followed by regular and ongoing contact with patients. During hospitalization when results of baseline polysomnography were reviewed, staff members provided patients with information about sleep apnea and CPAP. In the first month after in-home delivery of the CPAP machine, the patient was visited every week; thereafter, monthly telephone calls were made for the remainder of the 2-year study period. In-home visits were scheduled at 6, 12, and 24 months of CPAP use and at study end. During in-home visits, staff downloaded pressure, residual AHI, air leak, and compliance data from the CPAP machine and reviewed them with the patients. Staff members were also available to patients for ad hoc issues related to difficulties with mask fit or other technical problems with the CPAP machine.
4.4. Routine rehabilitation training
All patients received routine rehabilitation training as directed by the rehabilitation doctor, including treatment of cerebrovascular risk factors such as hypertension, diabetes, hyperlipidemia, and coronary disease. Physical and occupational therapies based on the Bobath technology were applied, such as joint activity training, induction of active body movement, balance training, position transfer training, and daily activity ability training. Total treatment duration was 6 months; physical and occupational therapies were performed once a day for 45 minutes, 5 days per week. Electrotherapy was performed once a day for 20 minutes and 5 days per week.
After 6 months of rehabilitation, the patient enters a chronic period, or a later period of recovery, and the community and outpatient department provide sequential rehabilitation. In this period, the patients were treated with targeted rehabilitation to prevent and treat the disability syndrome and improve daily activities. Regular outpatient service or home guidance was carried out by therapists to provide physical and occupational therapies.
All subjects were evaluated at admission, 6 months, 12 months, and 24 months follow-up for motor function by the Fugl-Meyer assessment scale (FMA), stroke severity by the National Institutes of Health Stroke Scale (NIHSS), cognitive function by Minimental state examination (MMSE), activities of daily life by the Barthel index (BI), psychological state by the Hamilton anxiety scale (HAMA) and Hamilton depression rating scale for depression (HRSD), and obesity by the BMI.
4.5. Sample size
According to Bravata DM,[30] the mean AHI would be 20.0 events/h with a standard deviation of 15.2 in the intervention group compared with 22.6 with a standard deviation of 16.1 in the control group, and according to Qaseem A,[31] the pre-post AHI were −19.85 events/h, CPAP vs no treatment. According to Menon D, the pre-post NIHSS were 11.03 and 8.15 in the OSA group, respectively. The trial was designed to recruit 40 patients in the CPAP group and 82 patients in the control group to have at least 80% statistical power to the two primary aims, the sample size estimates assumed a 10% loss to follow-up in both groups.[32]
4.6. Statistical analysis
Data analysis was performed with SPSS (version 22.0, IBM, New York, USA). Quantitative data are mean ± standard deviation (SD). Continuous variables were analyzed by Student's t test and categorical ones by the chi-square test or Fisher's exact test as applicable. The level of statistical significance was set at two tailed P < .05. Categorical data were presented as proportions and 95% confidence intervals (CIs). Descriptive statistics was used to characterize the sample in terms of demographic, clinical, sleep, and stroke variables. The CPAP and control groups were compared by independent samples Student's t test, and self-comparison to Entry (baseline) by paired-samples Student t test, and nonparametric test (χ2 test), as appropriate. Repeated-measures ANOVA was used to assess interactions between the two groups and changes. Different statistical methods were used to identify the effect of CPAP and rehabilitation on stroke recovery while keeping the effects of confounding factors at the minimum.
5. Results
5.1. Patient characteristics
During the study period, of the 398 patients with OSA admitted with acute stroke in our departments, 233 basal ganglia stroke cases (ischemic and hemorrhagic) were identified. After applying the selection criteria 146 were selected, of which 67 were later excluded: poor cooperation and incomplete data (n = 11) and withdrawal of consent (n = 9). Meanwhile, 18 patients were lost to follow-up (n = 18), including 2 patients in the CPAP group at 12 months, and 5 and 11 in the control group at 12 and 24 months, respectively. A total of 128 patients formed the final cohort (Fig. 1).
Figure 1.
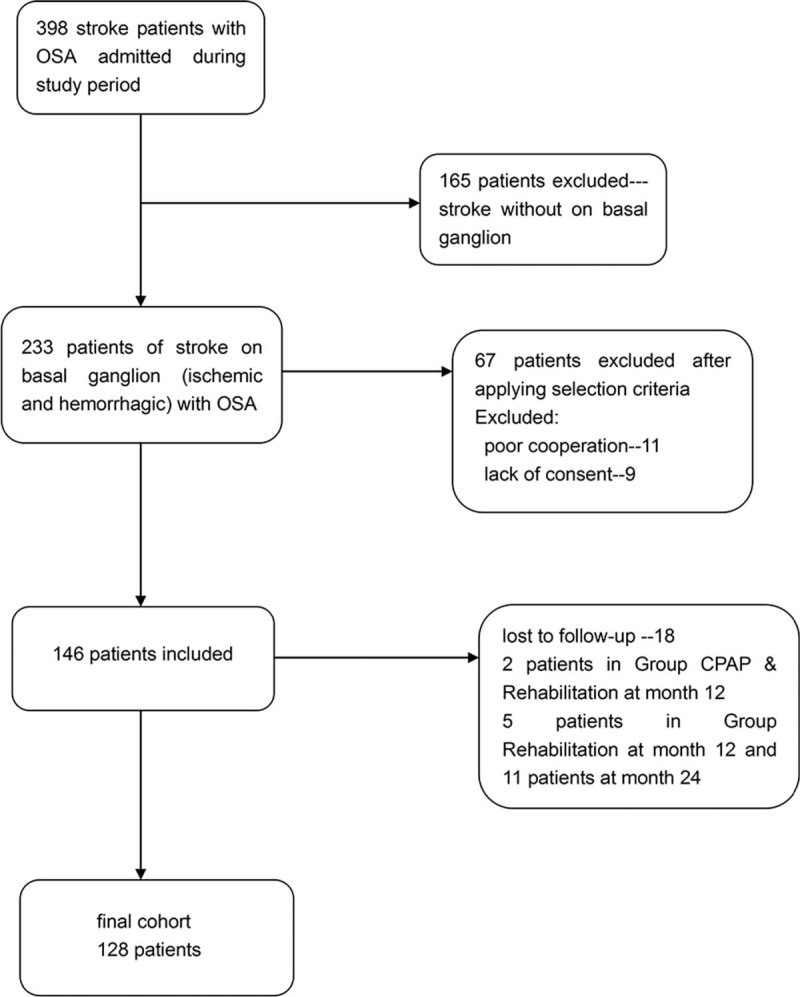
Flow chart showing the patients selection process leading to the final cohort.
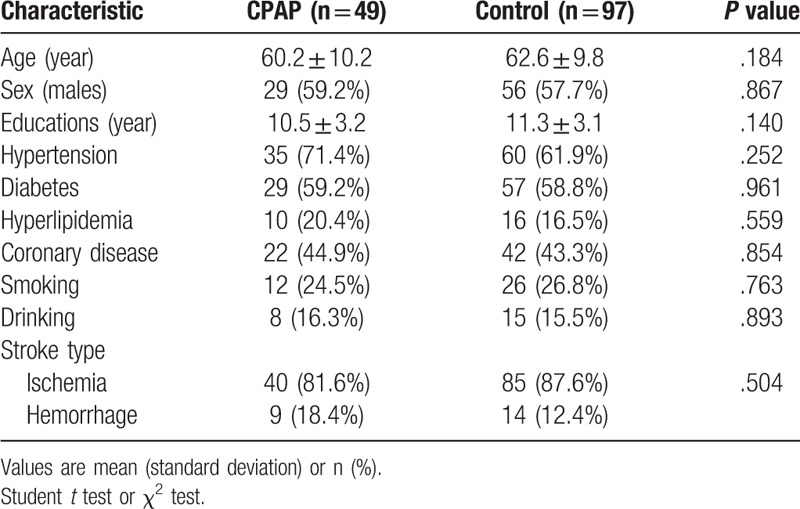
All baseline characteristics in the CPAP and control groups were compared (Table 1). There were no statistically significant differences in age, gender, smoking, drinking, education, hypertension, hyperlipidemia, diabetes, coronary artery disease, treatment with antiplatelet agents, and stroke type (ischemia or hemorrhage) between the two groups (all P > .05).
Table 1.
Baseline characteristics of participants in the CPAP and control groups.
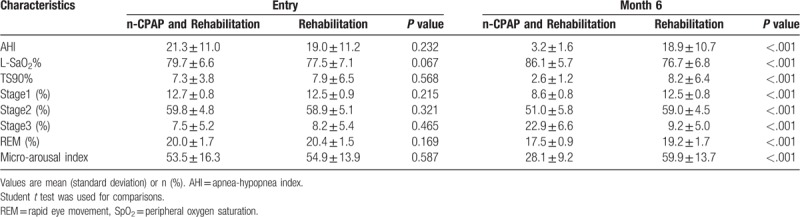
5.2. OSA-associated impairment of sleep parameters is improved with CPAP and rehabilitation in basal ganglia stroke patients
We examined the effects of OSA on sleep architecture. AHI and other respiratory parameters, such as minimum SpO2, percentage of time with SpO2 <90% and sleep architecture, were not significantly different between the two groups (P > .05) at baseline. At 6 months, the CPAP group had significantly lower mean AHI, TS90%, micro-arousal index, percentages of time in non-REM stages 1–2, and during the REM stage in total sleeping time compared with the control group, as well as markedly higher mean L-SaO2% and percentages of time in stage 3 (P < .001). Intermittent hypoxia at night was significantly improved by CPAP. There were no statistically significant differences in terms of AHI and other respiratory parameters between the two groups at 6 months (P > .05) (Table 2).
Table 2.
Respiratory parameters and sleep architecture in the CPAP and control groups.
5.3. CPAP and rehabilitation improve NIHSS, FMA, ADL, MMSE, HAMA, HRSD and BMI in basal ganglia stroke patients with OSA
There were no statistically significant difference in NIHSS, FMA, ADL, MMSE, HAMA, HRSD, and BMI at baseline between the CPAP and control groups (all P > .05) (Table 3).
Table 3.
Respiratory parameters and sleep architecture in the CPAP and control groups.
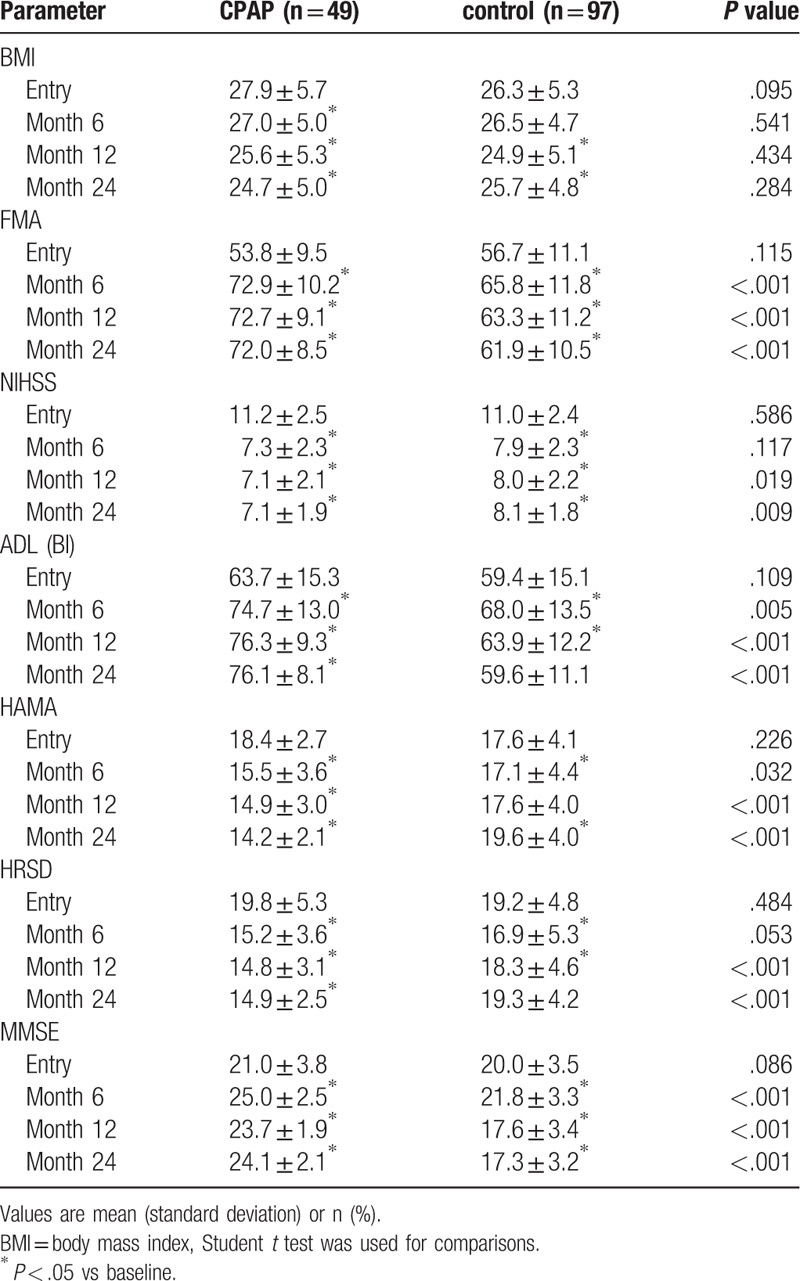
The CPAP group showed significant improvement in average FMA, ADL, MMSE, and HAMA scores at 6, 12, and 24 months compared with the control group (all P < .05). The CPAP group showed significant improvement in average NIHSS and HRSD scores at 12 and 24 months compared with the control group (P < .05). There was no difference in BMI management between the two groups (P > .05) (Table 3).
Repeated-measures ANOVA revealed significant interactions between the two groups and changes in NIHSS, FMA, ADL, MMSE, HAMA, and HRSD from baseline to 24 months (P < .001) (Figs. 2–7), but no significant interaction in BMI scores (P = .582) (Fig. 8).
Figure 2.
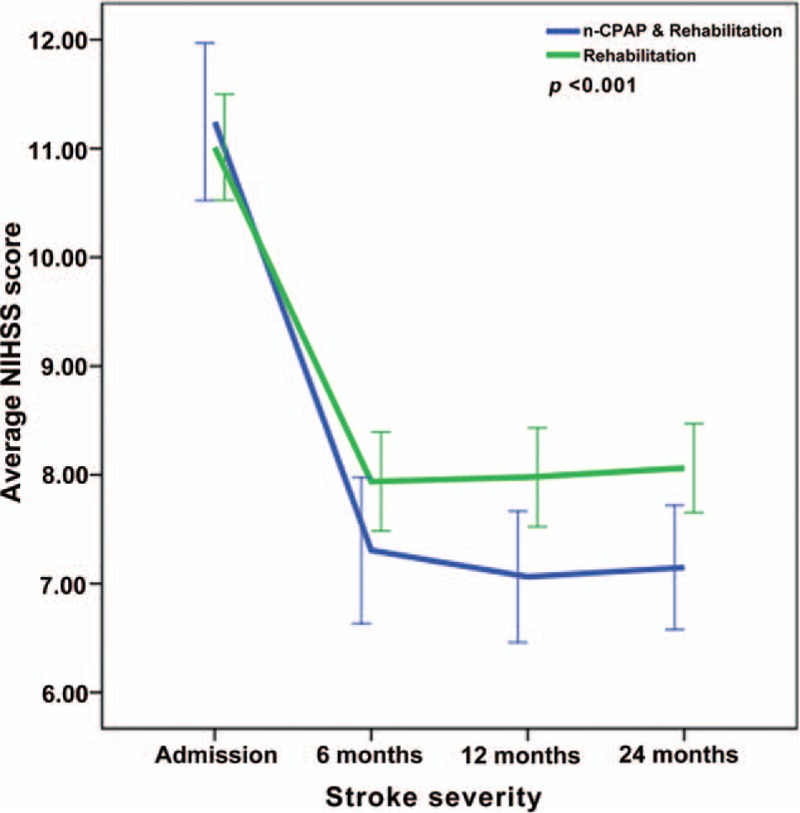
Repeated-measures ANOVA revealing significant interaction between the CPAP and control groups and NIHSS changes from baseline to twenty-four months (P < .001).
Figure 7.
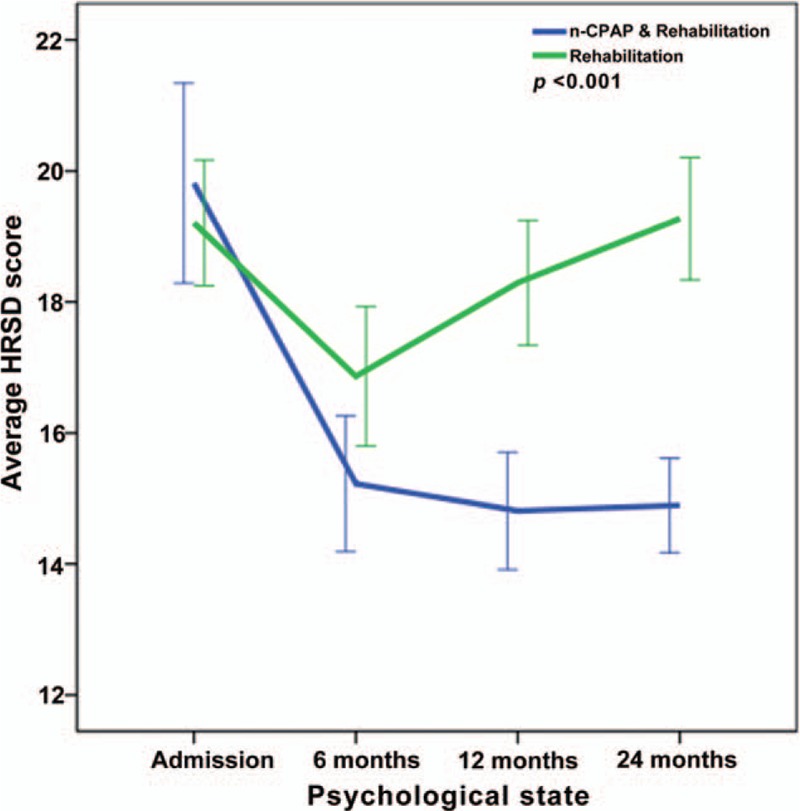
Repeated-measures ANOVA revealing significant interaction between the CPAP and control groups and HRSD changes from baseline to twenty-four months (P < .001).
Figure 8.
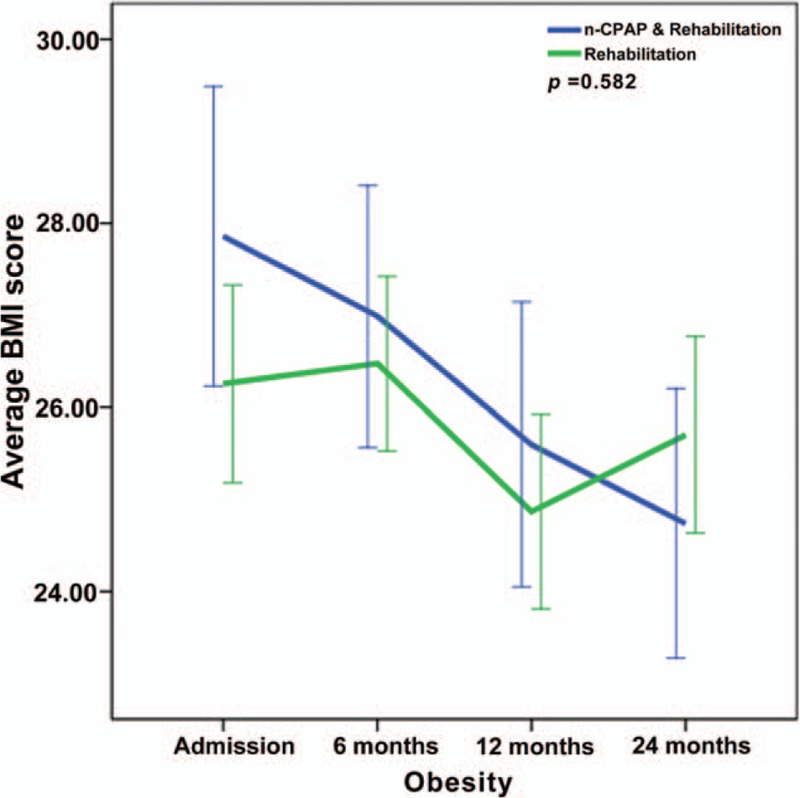
Repeated-measures ANOVA revealing significant interaction between the CPAP and control groups and BMI changes from baseline to twenty-four months (P = .582).
Figure 3.
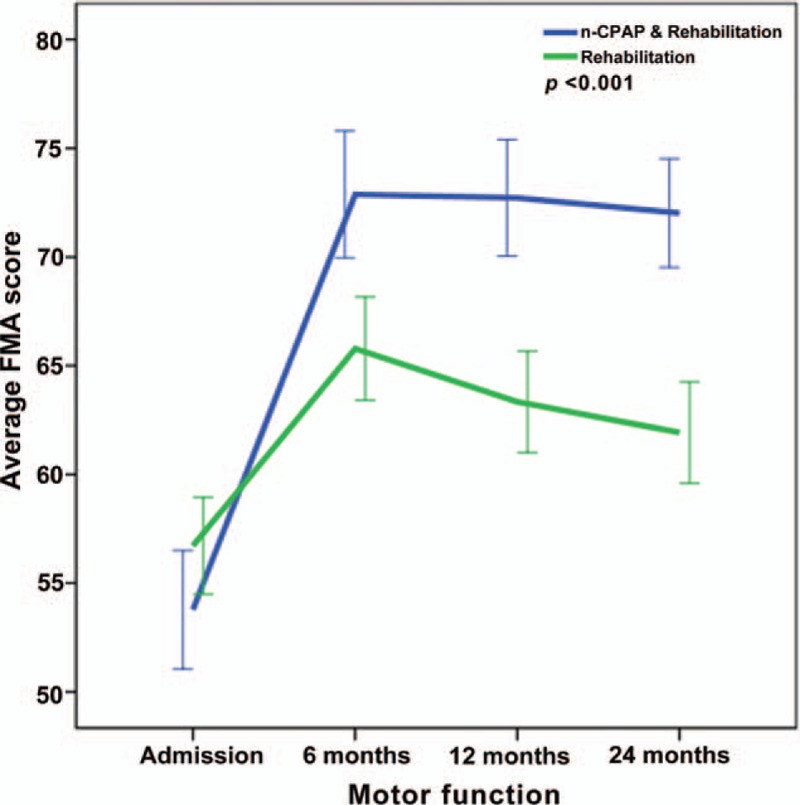
Repeated-measures ANOVA revealing significant interaction between the CPAP and control groups and FMA changes from baseline to twenty-four months (P < .001).
Figure 4.
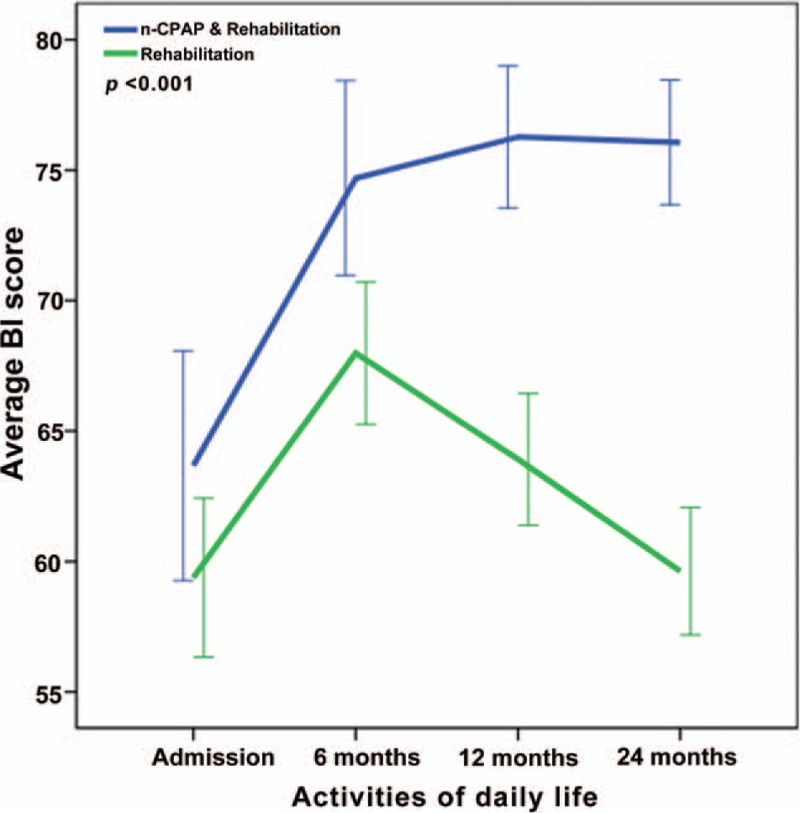
Repeated-measures ANOVA revealing significant interaction between the CPAP and control groups and BI changes from baseline to twenty-four months (P < .001).
Figure 5.
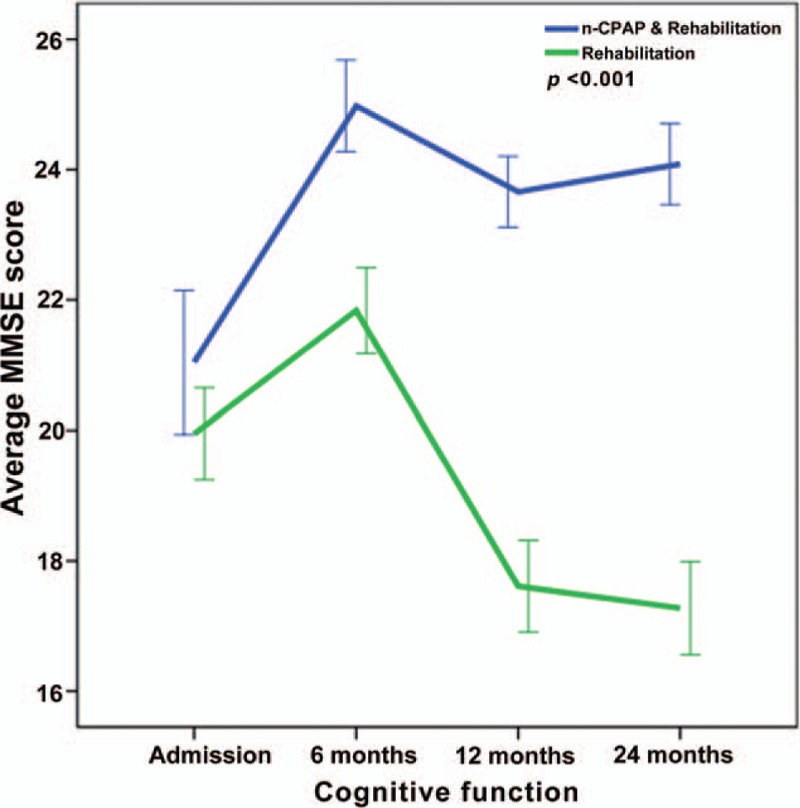
Repeated-measures ANOVA revealing significant interaction between the CPAP and control groups and MMSE changes from baseline to twenty-four months (P < .001).
Figure 6.
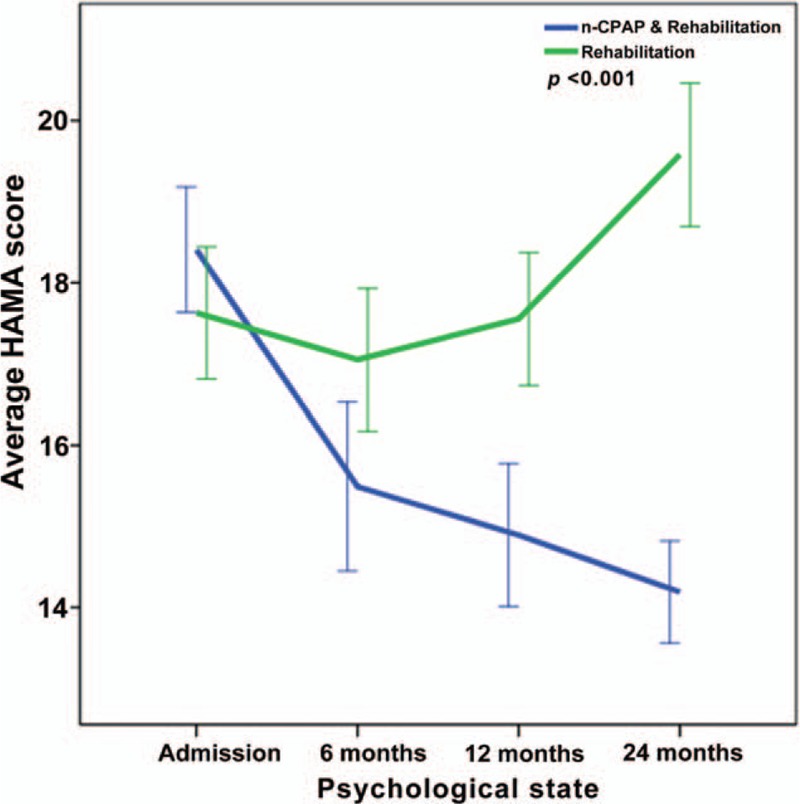
Repeated-measures ANOVA revealing significant interaction between the CPAP and control groups and HAMA changes from baseline to twenty-four months (P < .001).
6. Discussion
The prevalence of sleep-disordered breathing including OSA in the general population is variable, and ranges from 8% to 57% in men and 9% to 35% in women in various studies,[12,33,34] whereas in stroke cases, OSA prevalence ranges from 30% to as high as 80%.[19,35] In clinic, attention should be paid to stroke patients with OSA. This is the first prospective study focusing on the impact of CPAP and/or rehabilitation in stroke patients with OSA for 2 years. In the current cohort, intermittent hypoxia at night was significantly improved by CPAP. These results also demonstrated that a strategy of treating sleep apnea in patients with basal ganglia stroke could lead to substantial improvements of CPAP treatment.
The two groups had similar FMA and NIHSS at admission; however differences reached statistical significance at 6-month follow-up, and continued throughout the study. This is perhaps related to poor motor function and stroke severity improvement in the control group compared with the CPAP group. This suggests that CPAP and early rehabilitation training in stroke patients with OSA may obviously improve motor function and disability, increasing the quality of life.
By comparing the degree of improvement in terms of BI score change by 1 or more, we found that compared with the CPAP group, patients with rehabilitation only performed significantly less in recovery, revealing that rehabilitation has significantly lesser chance of improvement in the activities of daily living. However, the SAVE study assessed the possible benefits of CPAP in preventing cardiovascular events in OSA, and obtained less encouraging outcome.[36] Patients with moderate to severe OSA combined with coronary or cerebrovascular diseases treated with CPAP showed no benefit compared with those on routine care in terms of prevention of cardiovascular events. The relatively short compliance period for CPAP and a delay between implementing CPAP after identification of coronary and cerebrovascular events may be potential factors that require further investigation. As a rehabilitation center for advanced neurological and respiratory services, it is possible that more patients with both respiratory and neurological issues could have been referred to our center. Future studies should focus on whether early detection of OSA in stroke patients could translate into clinical benefit by CPAP application to assist stroke recovery.
OSA was reported to exaggerate cognitive dysfunction in stroke patients, which potentially impairs rehabilitation.[37,38] Yan Zhang clarified that OSA is an independent risk factor in addition to stroke for impairing cognitive function (e.g., prospective memory) in stroke patients.[39] As a potential underlying pathogenic mechanism, OSA decreases oxygen supply to the brain; meanwhile, stroke, a major brain disorder, often damages cognitive function.[40] In previous studies, OSA has been associated with severer cognitive dysfunction in stroke patients, and the pattern of OSA-related cognitive impairment differs from typical stroke-caused cognitive deficits. We found improvement of cognitive dysfunction in stroke patients with OSA, which indicates that both CPAP and rehabilitation are two complementary measures. Indeed, using CPAP was more efficient than rehabilitation alone as shown above. A number of previous studies have associated OSA-related cognitive dysfunction with hypoxia.[41–43] In patients with insomnia, sleep discontinuity was shown to damage attention and episodic memory.[44] It should be noted that in both stroke and snoring patients, OSA tends to shorten sleeping time at the slow wave stage (SWS), which is essential in the consolidation of declarative memory in young and healthy people.[45–48] As a potential mechanism, the active behavior first generates labile information storage in the hippocampus; during SWS, this temporally encoded hippocampal information is reactivated and transmitted to the cerebral cortex and integrated into a more permanent memory.[49] In stroke patients, SWS activity increases over the infarct area and decreases in the peri-infarct area.[50] Moreover, arteriolosclerosis and subcortical infarcts in older adults are associated with sleep fragmentation.[51] Most importantly, OSA is a treatable disorder. CPAP therapy has been shown to improve episodic memory, attention and executive functioning in non-stroke OSA patients.[52–54] Recently, similar therapeutic effects were also observed in stroke patients suffering of OSA.[38] CPAP and other anti-OSA therapies also improve sleep efficiency and reduce sleep fragmentation.[53,55]
A long-term home-based programmer of aerobic physical activity improves metabolic asset and reduces systemic inflammation in sedentary people of many diseases, such as these of atherosclerotic-based, degenerative and neoplastic.[56] However, it should also be noted that the beneficial effects of CPAP and rehabilitation in stroke patients are more pronounced than those of rehabilitation alone, which might be due to the large capacity of stroke patients with OSA than that of stroke patients to compensate OSA-induced neuronal degeneration and mood disorder. Accordingly, typical hallmarks of this disorder are sleep disruption and non-restorative sleep induced by frequent occurrence of upper airway obstruction. These nocturnal impairments can lead to daytime consequences. As a result, OSA patients frequently complain of excessive daytime sleepiness,[57] tiredness, insomnia, anxiety, stress-related disorder,[58] and, in some cases, depressive symptoms.[59,60] In this study, stroke patients with OSA suffered from psychological disorders at various degrees. We found a significantly improvement in the psychological state in the CPAP group compared with patients receiving rehabilitation alone, indicating that continued adaptation to CPAP may keep the mental state stable.
Obesity is an important risk factor for OSA, and weight loss reduces apnea severity, and even leads to resolution in some patients. However, CPAP is associated with weight gain in some patients but never weight loss; indeed, BMI increases with 1 year of CPAP use in women but not men and in non-obese subjects.[61] In the majority of CPAP-treated OSA patients, weight did not significantly change but increased slightly slower than in the age-matched general population. However, in 10% of patients, high adherence to CPAP treatment did not prevent further weight gain. These patients present a high-risk group for OSA-related multi-morbidity later in life.[62] OSA patients treated with CPAP may gain a modest amount of weight, with the greatest weight gain found in individuals most compliant to CPAP.[63] However, the latter study failed to address a key aspect—whether the weight gain is due to adipose tissue or lean body mass (LBM).[64] However, effective CPAP therapy may lead to weight loss by any of several proposed mechanisms, including, but not limited to increased physical activity and enhanced responsiveness to leptin. The role of leptin in the development of OSA has been reported. However, the effects of OSA treatment using CPAP on serum leptin levels remain controversial. After 6 months of CPAP or surgery, leptin, IL-6 and TNF-α levels are decreased in all OSA patients,[65] but no changes in leptin were detected after 6 months of CPAP in OSA patients in another study.[66] Evidence from a meta-analysis assessing the effect CPAP therapy on decreasing leptin levels in OSA patients is low, and stronger evidence is needed.[67] In this study, we postulate that weight management would benefit overweight/obese OSA patients with stroke, with addition of upper airway management, such as with CPAP. However, as the review above clarifies, both CPAP and weight loss independently lead to improvement in OSA severity and stroke recovery.
This study had many merits in that it was a well-planned, supervised, prospective study, with long-term follow-up. In addition, we employed AASM criteria to diagnose OSA. However, many studies were based on oximetry alone, which is inferior to PSG or even portable monitors.[68,69] There are several limitations of this study as well. First, compliance to CPAP treatment is too low and could be randomized in this study. Secondly, sample size was relatively small and no blinding was used, and the sample size of hemorrhagic strokes was too small to compare the differences of outcome between ischemic and hemorrhagic strokes with CPAP therapy. Thirdly, there might be selection bias in the patient population with basal ganglia stroke (both ischemic and hemorrhagic). It is assumed that only patients with stroke on the same location visited our hospitals. In addition, the evaluated indicators, such as FMA, ADL, MMSE, HAMA, and HRSD, were scales, which are not as objective as PSG parameters.
7. Conclusion
This study demonstrated that OSA significantly and independently contributes to neurological and cognitive dysfunction in Chinese stroke patients, which is associated with hypoxemia and disrupted sleep. In stroke patients with OSA, CPAP and routine rehabilitation training might improve cognitive function, facilitate motor function recovery and increase the quality of life. In the context of increasing recognition of OSA as a potentially modifiable risk factor for primary as well as secondary stroke prevention, the present study highlighted the importance of intervention for OSA, not only for the prevention of stroke recurrence, but also for potential enhancement of post-stroke recovery.
Acknowledgments
This study was supported by research grants from the National Science Foundation of China (NO: NSFC81770085), Suzhou Special Project of Diagnosis and Therapeutics for Clinical Key Diseases (NO: LCZX201604), the Key Construction Projects of Shanghai Health and Family Planning on Weak Discipline (NO: 2015ZB0401), Research projects of Jingan District Health and Family Planning Commission, Shanghai (NO: 2018MS21), and Research projects of Shanghai Health and Family Planning Commission (NO: 201540060).
Author contributions
Conceptualization: Rui Chen.
Investigation: Lei Ren, Kai Wang, Honghua Shen, Yiming Xu, Jing Wang.
Methodology: Lei Ren, Kai Wang, Honghua Shen, Yiming Xu, Jing Wang.
Writing – original draft: Lei Ren.
Writing – review & editing: Rui Chen.
Footnotes
Abbreviations: AHI = apnea-hypopnea index, Ang II = angiotensin II, BI = Barthel index, CPAP = continuous positive airway pressure, FMA = fugl-Meyer assessment scale, FVII = factor VII, FVIIa-AT = factor VIIa-antithrombin complexes, HAMA = hamilton anxiety scale, HRSD = hamilton depression rating scale for depression, LBM = lean body mass, MMSE = minimental state examination, NIHSS = health Stroke Scale, non-REM = non-rapid eye movement, OSA = obstructive sleep apnea, PAI-1 = plasminogen activator inhibitor-1, PSG = polysomnography, RAAS = renin-angiotensin-aldosterone system, REM = rapid eye movement, SWS = slow wave stage, TF = total tissue factor.
The authors declare that there is no conflict of interest.
References
- [1].Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 2014;383:245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rothwell PM. AVERT: a major milestone in stroke research. Lancet 2015;386:7–9. [DOI] [PubMed] [Google Scholar]
- [3].Krishnamurthi RV, Feigin VL, Forouzanfar MH, et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health 2013;1:e259–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Slomka A, Switonska M, Sinkiewicz W, et al. Assessing circulating factor VIIa-antithrombin complexes in acute ischemic stroke: a pilot study. Clin Appl Thromb Hemost 2017;23:351–9. [DOI] [PubMed] [Google Scholar]
- [5].Di Raimondo D, Tuttolomondo A, Butta C, et al. Effects of ACE-inhibitors and angiotensin receptor blockers on inflammation. Curr Pharm Des 2012;18:4385–413. [DOI] [PubMed] [Google Scholar]
- [6].Licata G, Tuttolomondo A, Corrao S, et al. Immunoinflammatory activation during the acute phase of lacunar and non-lacunar ischemic stroke: association with time of onset and diabetic state. Int J Immunopathol Pharmacol 2006;19:639–46. [DOI] [PubMed] [Google Scholar]
- [7].Tuttolomondo A, Di Sciacca R, Di Raimondo D, et al. Effects of clinical and laboratory variables and of pretreatment with cardiovascular drugs in acute ischaemic stroke: a retrospective chart review from the GIFA study. Int J Cardiol 2011;151:318–22. [DOI] [PubMed] [Google Scholar]
- [8].Winstein CJ, Stein J, Arena R, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2016;47:e98–169. [DOI] [PubMed] [Google Scholar]
- [9].Loke YK, Brown JW, Kwok CS, et al. Association of obstructive sleep apnea with risk of serious cardiovascular events: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes 2012;5:720–8. [DOI] [PubMed] [Google Scholar]
- [10].Labarca G, Cruz NR, Descalzi F. Multisystemic involvement in obstructive sleep apnea. Rev Med Chil 2014;142:748–57. [DOI] [PubMed] [Google Scholar]
- [11].Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis 2015;7:1311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Arnardottir ES, Bjornsdottir E, Olafsdottir KA, et al. Obstructive sleep apnoea in the general population: highly prevalent but minimal symptoms. Eur Respir J 2016;47:194–202. [DOI] [PubMed] [Google Scholar]
- [13].Valham F, Mooe T, Rabben T, et al. Increased risk of stroke in patients with coronary artery disease and sleep apnea: a 10-year follow-up. Circulation 2008;118:955–60. [DOI] [PubMed] [Google Scholar]
- [14].Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005;353:2034–41. [DOI] [PubMed] [Google Scholar]
- [15].Rivas M, Ratra A, Nugent K. Obstructive sleep apnea and its effects on cardiovascular diseases: a narrative review. Anatol J Cardiol 2015;15:944–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kimoff RJ. Sleep fragmentation in obstructive sleep apnea. Sleep 1996;199 Suppl:S61–6. [DOI] [PubMed] [Google Scholar]
- [17].American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed.Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- [18].Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med Rev 2017;34:70–81. [DOI] [PubMed] [Google Scholar]
- [19].Johnson KG, Johnson DC. Frequency of sleep apnea in stroke and TIA patients: a meta-analysis. J Clin Sleep Med 2010;6:131–7. [PMC free article] [PubMed] [Google Scholar]
- [20].Kaneko Y, Hajek VE, Zivanovic V, et al. Relationship of sleep apnea to functional capacity and length of hospitalization following stroke. Sleep 2003;26:293–7. [DOI] [PubMed] [Google Scholar]
- [21].Yan-fang S, Yu-ping W. Sleep-disordered breathing: impact on functional outcome of ischemic stroke patients. Sleep Med 2009;10:717–9. [DOI] [PubMed] [Google Scholar]
- [22].King S, Cuellar N. Obstructive sleep apnea as an independent stroke risk factor: a review of the evidence, stroke prevention guidelines, and implications for neuroscience nursing practice. J Neurosci Nurs 2016;48:133–42. [DOI] [PubMed] [Google Scholar]
- [23].Colten HR, Altevogt BM. Institute of Medicine Committee on Sleep M, Research. The National Academies Collection: Reports funded by National Institutes of Health. National Academies Press (US) National Academy of Sciences, Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington (DC): 2006. [PubMed] [Google Scholar]
- [24].Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:2160–236. [DOI] [PubMed] [Google Scholar]
- [25].Inoue A, Chiba S, Matsuura K, et al. Nasal function and CPAP compliance. Auris Nasus Larynx 2018;46:548–58. [DOI] [PubMed] [Google Scholar]
- [26].Zhu D, Wu M, Cao Y, et al. Heated humidification did not improve compliance of positive airway pressure and subjective daytime sleepiness in obstructive sleep apnea syndrome: A meta-analysis. PLoS One 2018;13:e0207994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ma Z, Drinnan M, Hyde P, et al. Mask interface for continuous positive airway pressure therapy: selection and design considerations. Expert Rev Med Devices 2018;15:725–33. [DOI] [PubMed] [Google Scholar]
- [28].Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2012;8:597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hirshkowitz M, Littner M, Kuna S, et al. Sleep-related breathing disorders: sourcebook. 2nd ed.Milwaukee: HAIG; 2003. [Google Scholar]
- [30].Bravata DM, McClain V, Austin C, et al. Diagnosing and managing sleep apnea in patients with chronic cerebrovascular disease: a randomized trial of a home-based strategy. Sleep Breath 2017;21:713–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Qaseem A, Holty JE, Owens DK, et al. Management of obstructive sleep apnea in adults: A clinical practice guideline from the American College of Physicians. Ann Intern Med 2013;159:471–83. [DOI] [PubMed] [Google Scholar]
- [32].Menon D, Sukumaran S, Varma R, et al. Impact of obstructive sleep apnea on neurological recovery after ischemic stroke: A prospective study. Acta Neurol Scand 2017;136:419–26. [DOI] [PubMed] [Google Scholar]
- [33].Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc 2008;5:136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tufik S, Santos-Silva R, Taddei JA, et al. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med 2010;11:441–6. [DOI] [PubMed] [Google Scholar]
- [35].Mirrakhimov AE, Sooronbaev T, Mirrakhimov EM. Prevalence of obstructive sleep apnea in Asian adults: a systematic review of the literature. BMC Pulm Med 2013;13:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016;375:919–31. [DOI] [PubMed] [Google Scholar]
- [37].Aaronson JA, van Bennekom CA, Hofman WF, et al. Obstructive sleep apnea is related to impaired cognitive and functional status after stroke. Sleep 2015;38:1431–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Aaronson JA, Hofman WF, van Bennekom CA, et al. Effects of continuous positive airway pressure on cognitive and functional outcome of stroke patients with obstructive sleep apnea: a randomized controlled trial. J Clin Sleep Med 2016;12:533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhang Y, Wang W, Cai S, et al. Obstructive sleep apnea exaggerates cognitive dysfunction in stroke patients. Sleep Med 2017;33:183–90. [DOI] [PubMed] [Google Scholar]
- [40].Levine DA, Galecki AT, Langa KM, et al. Trajectory of cognitive decline after incident stroke. JAMA 2015;314:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 2011;306:613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gelber RP, Redline S, Ross GW, et al. Associations of brain lesions at autopsy with polysomnography features before death. Neurology 2015;84:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Launer LJ, Hughes TM, White LR. Microinfarcts, brain atrophy, and cognitive function: the Honolulu Asia Aging Study Autopsy Study. Ann Neurol 2011;70:774–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fortier-Brochu E, Morin CM. Cognitive impairment in individuals with insomnia: clinical significance and correlates. Sleep 2014;37:1787–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Deliens G, Leproult R, Neu D, et al. Rapid eye movement and non-rapid eye movement sleep contributions in memory consolidation and resistance to retroactive interference for verbal material. Sleep 2013;36:1875–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Cedernaes J, Rangtell FH, Axelsson EK, et al. Short sleep makes declarative memories vulnerable to stress in humans. Sleep 2015;38:1861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Griessenberger H, Hoedlmoser K, Heib DP, et al. Consolidation of temporal order in episodic memories. Biol Psychol 2012;91:150–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Scullin MK. Sleep, memory, and aging: the link between slow-wave sleep and episodic memory changes from younger to older adults. Psychol Aging 2013;28:105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mitra A, Snyder AZ, Hacker CD, et al. Human cortical-hippocampal dialogue in wake and slow-wave sleep. Proc Natl Acad Sci U S A 2016;113:E6868–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Poryazova R, Huber R, Khatami R, et al. Topographic sleep EEG changes in the acute and chronic stage of hemispheric stroke. J Sleep Res 2015;24:54–65. [DOI] [PubMed] [Google Scholar]
- [51].Lim AS, Yu L, Schneider JA, et al. Sleep fragmentation, cerebral arteriolosclerosis, and brain infarct pathology in community-dwelling older people. Stroke 2016;47:516–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Dalmases M, Sole-Padulles C, Torres M, et al. Effect of CPAP on cognition, brain function, and structure among elderly patients with OSA: a randomized pilot study. Chest 2015;148:1214–23. [DOI] [PubMed] [Google Scholar]
- [53].Kushida CA, Nichols DA, Holmes TH, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: The Apnea Positive Pressure Long-term Efficacy Study (APPLES). Sleep 2012;35:1593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Castronovo V, Scifo P, Castellano A, et al. White matter integrity in obstructive sleep apnea before and after treatment. Sleep 2014;37:1465–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hetzenecker A, Escourrou P, Kuna ST, et al. Treatment of sleep apnea in chronic heart failure patients with auto-servo ventilation improves sleep fragmentation: a randomized controlled trial. Sleep Med 2016;17:25–31. [DOI] [PubMed] [Google Scholar]
- [56].Di Raimondo D, Tuttolomondo A, Butta C, et al. Metabolic and anti-inflammatory effects of a home-based programme of aerobic physical exercise. Int J Clin Pract 2013;67:1247–53. [DOI] [PubMed] [Google Scholar]
- [57].Seneviratne U, Puvanendran K. Excessive daytime sleepiness in obstructive sleep apnea: prevalence, severity, and predictors. Sleep Med 2004;5:339–43. [DOI] [PubMed] [Google Scholar]
- [58].Amdo T, Hasaneen N, Gold MS, et al. Somatic syndromes, insomnia, anxiety, and stress among sleep disordered breathing patients. Sleep Breath 2016;20:759–68. [DOI] [PubMed] [Google Scholar]
- [59].Harris M, Glozier N, Ratnavadivel R, et al. Obstructive sleep apnea and depression. Sleep Med Rev 2009;13:437–44. [DOI] [PubMed] [Google Scholar]
- [60].Bjornsdottir E, Benediktsdottir B, Pack AI, et al. The prevalence of depression among untreated obstructive sleep apnea patients using a standardized psychiatric interview. J Clin Sleep Med 2016;12:105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Redenius R, Murphy C, O’Neill E, et al. Does CPAP lead to change in BMI? J Clin Sleep Med 2008;4:205–9. [PMC free article] [PubMed] [Google Scholar]
- [62].Myllyla M, Kurki S, Anttalainen U, et al. High adherence to CPAP treatment does not prevent the continuation of weight gain among severely obese OSAS patients. J Clin Sleep Med 2016;12:519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Quan SF, Budhiraja R, Clarke DP, et al. Impact of treatment with continuous positive airway pressure (CPAP) on weight in obstructive sleep apnea. J Clin Sleep Med 2013;9:989–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Mysliwiec V, O’Reilly B, Roth BJ. Weight gain with CPAP: a complication of treatment? J Clin Sleep Med 2014;10:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].De Santis S, Cambi J, Tatti P, et al. Changes in ghrelin, leptin and pro-inflammatory cytokines after therapy in Obstructive Sleep Apnea Syndrome (OSAS) patients. Otolaryngol Pol 2015;69:1–8. [DOI] [PubMed] [Google Scholar]
- [66].Castro-Grattoni AL, Torres G, Martinez-Alonso M, et al. Blood pressure response to CPAP treatment in subjects with obstructive sleep apnoea: the predictive value of 24-h ambulatory blood pressure monitoring. Eur Respir J 2017;50: [DOI] [PubMed] [Google Scholar]
- [67].Zhang P, Liu J, Long S, et al. Association between continuous positive airway pressure and changes in serum leptin in patients with obstructive sleep apnoea: a meta-analysis. Sleep Breath 2014;18:695–702. [DOI] [PubMed] [Google Scholar]
- [68].Cherkassky T, Oksenberg A, Froom P, et al. Sleep-related breathing disorders and rehabilitation outcome of stroke patients: a prospective study. Am J Phys Med Rehabil 2003;82:452–5. [PubMed] [Google Scholar]
- [69].Nickerson J, Lee E, Nedelman M, et al. Feasibility of portable sleep monitors to detect obstructive sleep apnea (OSA) in a vulnerable urban population. J Am Board Fam Med 2015;28:257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]


