Table 5. One-Pot Synthesis of 4H-Pyran Derivatives in Refluxing Ethanol.
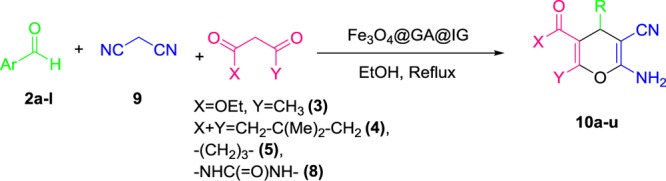
| entry | aldehyde | 1,3-dicarbonyl | product | time (min) | yield (%) | mp (°C) | lit. mp (°C) |
|---|---|---|---|---|---|---|---|
| 1 | 4-chlrobenzaldehyde | 3 | 10a | 60 | 90 | 172–174 | 174–17527 |
| 2 | 4-nitrobenzaldehyde | 3 | 10b | 60 | 92 | 182–186 | 183–18527 |
| 3 | 4-methylbenzaldehyde | 3 | 10c | 95 | 82 | 175–177 | 177–17927 |
| 4 | 4-methoxybenzaldehyde | 3 | 10d | 110 | 78 | 135–137 | 136–13727 |
| 5 | 3-nitrobenzaldehyde | 3 | 10e | 15 | 95 | 199–202 | 198–20027 |
| 6 | 4-chlrobenzaldehyde | 4 | 10f | 15 | 92 | 214–216 | 215–21628 |
| 7 | 4-cyanobenzaldehyde | 4 | 10g | 20 | 90 | 229–231 | 228–22927 |
| 8 | 3-nitrobenzaldehyde | 4 | 10h | 20 | 90 | 216–218 | 214–21629 |
| 9 | 4-methoxybenzaldehyde | 4 | 10i | 50 | 85 | 200–203 | 201–20227 |
| 10 | furfural | 4 | 10j | 90 | 80 | 221–224 | 221–22430 |
| 11 | 4-chlrobenzaldehyde | 5 | 10k | 20 | 91 | 222–225 | 223–22630 |
| 12 | 4-cyanobenzaldehyde | 5 | 10l | 30 | 90 | 237–238 | 235–23731 |
| 13 | 3-nitrobenzaldehyde | 5 | 10m | 35 | 89 | 197–200 | 200–20220 |
| 14 | 4-methoxybenzaldehyde | 5 | 10n | 90 | 83 | 206–209 | 207–20932 |
| 15 | furfural | 5 | 10o | 85 | 81 | 236–239 | 237–23930 |
| 16 | thiophene-2-carbaldehyde | 5 | 10p | 40 | 96 | 223–224 | 223–22542 |
| 17 | 4-chlrobenzaldehyde | 8 | 10q | 25 | 90 | 232–236 | 234–23629 |
| 18 | 2-nitrobenzaldehyde | 8 | 10r | 30 | 91 | 257–258 | 255–25729 |
| 19 | benzaldehyde | 8 | 10s | 40 | 88 | 208–210 | 209–21034 |
| 20 | 4-methylbenzaldehyde | 8 | 10t | 50 | 85 | 228–230 | 226–22734 |
| 21 | 4-methoxybenzaldehyde | 8 | 10u | 65 | 89 | 282–283 | 280–28134 |
