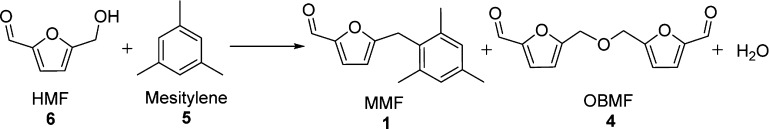
Table 2. Arylation of HMF with Mesitylene over Various Catalystsa.
| Conv.c (%) | yieldd (%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| entry | catalyst | loading | T (°C) | t (h) | 6 | 1 | 4 | TOF (h–1) |
| 1 | concd H2SO4 | 10 mol % | 80 | 3 | >99 | 75 | 11 | 0.2 |
| 2 | Amberlyst-15 | 0.1 g | 80 | 4 | >99 | 79 | 07 | 13.83 |
| 3 | ZrOCl2·8H2O | 0.1 g | 80 | 16 | >99 | 30 | 11 | 0.14 |
| 4 | Mont | 0.1 g | 110 | 16 | 44 | 21 | 09 | 2.5 |
| 5 | Zr-Mont | 0.1 g | 110 | 12 | >99 | 86 | 02 | 2.63 |
| 6 | Zr-Mont | 0.05 g | 110 | 16 | 64 | 50 | 00 | 3.15 |
| 7 | Zr-Mont | 0.2 g | 110 | 16 | >99 | 75 | 17 | 1.57 |
| 8 | Sn-Mont | 0.1 g | 110 | 16 | >99 | 70 | 21 | 2.09 |
| 9 | Al-Mont | 0.1 g | 110 | 16 | 72 | 57 | trace | 2.70 |
| 10 | Fe-Mont | 0.1 g | 110 | 16 | 55 | 43 | 2.79 | |
| 11b | Zr-Mont | 0.1 g | 110 | 16 | >99 | 83 | 02 | 2.67 |
Reaction conditions: HMF (0.252 g, 2 mmol), mesitylene (5 mL), nitroethane (5 mL), and catalyst.
Crude HMF obtained from fructose.
Conversion of HMF was measured using HPLC.
Isolated yields.