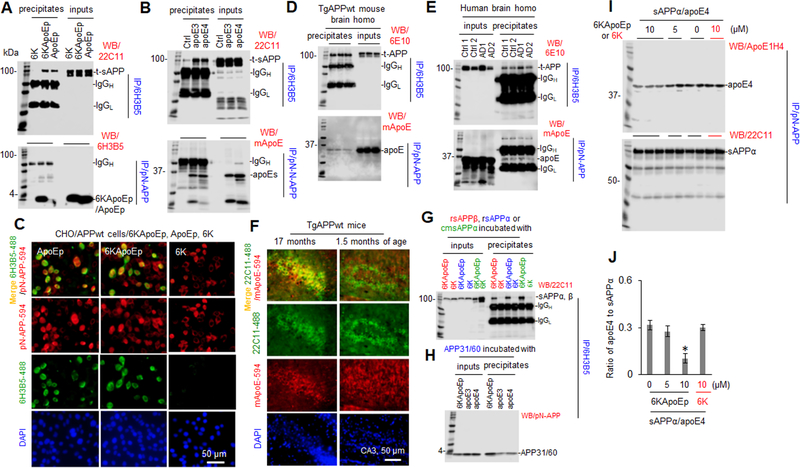
Figure 2. 6KApoEp physically interacts with N-terminal APP.
CHO/APPwt cells were treated with 6KApoEp, ApoEp, or 6K at 10 μM (A), apoE3 or 4 at 10 μg/mL, or PBS control (Ctrl, B) for 2 h followed by immunoprecipitation (IP) of the conditioned media with mouse monoclonal anti-apoE LDL binding domain antibody (6H3B5) or rabbit polyclonal anti-N-terminal APP41/66 antibody (pN-APP). Total secreted APP (t-sAPP), 6KApoEp, ApoEp, apoE3, and apoE4 4 in total conditioned media (inputs) and immunoprecipitates (precipitates) was then determined by WB using mouse anti-N-terminal APP antibody (22C11), 6H3B5, and rabbit anti-human apoE antibody (mApoE) (A, B), respectively. CHO/APPwt cells were also plated at 8 well-chambers at 1 × 105/well for 24 h, treated with 6K, ApoEp, or 6KApoEp at 10 μM for 2 h, fixed in 4% paraformaldehyde solution and stained with 6H3B5 and pN-APP (C). Alexa Fluor 488 goat anti-mouse IgG was used to detect ApoEp and 6KApoEp (green), while Alexa Fluor 594 donkey anti-rabbit IgG was used to detect N-terminal APP (red). DAPI costaining showed nuclear DNA. Brain tissue homogenates prepared from three TgAPPwt mice (two female/one male), two AD patients (AD1/male, AD2/female) and two normal age-matched control cortices (Ctrl1/male, Ctrl2/female) were IP with 6H3B5 or pN-APP and t-APP and apoE were determined by WB analysis using 6E10 and mApoE, respectively (D, E). Brain tissue sections from aged and young TgAPPwt mice (n = two for each group, one female/one male) were also stained with 22C11 and mApoE at 4°C overnight, followed by staining with Alexa Fluor 488 goat anti-mouse IgG to detect cell surface APP (green) and Alexa Fluor 594 donkey anti-rabbit IgG to detect apoE (red, F). As in vitro confirmation, human recombinant sAPPβ (rsAPPβ, without Aβ domain), rsAPPα, CHO/APPwt cell conditioned media-derived sAPPα (cmsAPPα), or APP N-terminal peptide (aa 31–60, APP31/60) at 100 nM was incubated with 6KApoEp, 6K, apoE3, or apoE4 at 100 nM, at 37°C for 1 h followed by IP with 6H3B5. sAPPα/β and APP31/60 in total (inputs) and immunoprecipitates were determined by WB analysis using 22C11and pN-APP, respectively (G, H). In addition, human sAPPα protein at 100 nM was also incubated with apoE4 protein at 100 nM in the absence or presence of 6KApoEp or 6K at 5 or 10 μM for 1 h at 37°C, followed by IP with pN-APP and determination of apoE4 and sAPPα in precipitates by WB analysis using ApoE1H4 and 22C11, respectively (I). Band density ratios of apoE4 to total sAPPα was determined by densitometry analysis (J). The results shown in J panel are representative of two independent experiments with each condition duplicated. Asterisk indicates p < 0.05 compared with the controls as determined by t-test. Overall, 6KApoEp, ApoEp, apoE3, and apoE4, but not 6K, were immunoprecipitated and localized with N-terminal APP in vitro and in vivo. ApoE was co-localized with N-terminal APP more in aged compared with young TgAPPwt mouse brains. 6KApoEp, but not 6K, reduced co-immunoprecipitation of sAPPα with apoE4. AD patient and age-matched control cortices provided by Banner Sun Health Research Institute (Sun City, AZ). The results shown in A-D panels are representative of two to three independent experiments.