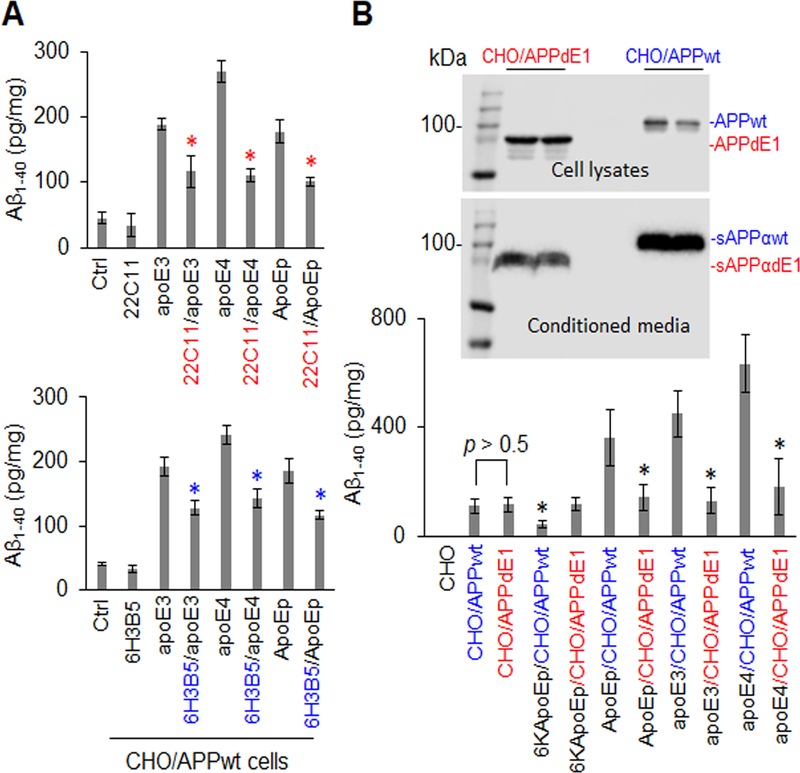
Figure 4. ApoE-promoted Aβ production was significantly attenuated by specific antibodies against the apoE LDLR binding domain or the N-terminal APP or by expression of N-terminally truncated APP.
CHO/APPwt cells were treated with apoE3 or apoE4 at 10 mg/mL or ApoEp at 5 μM in the absence or presence of anti-N-terminal APP (22C11) or anti-apoE LDLR binding domain antibody (6H3B5) at 10 μg/mL for 2 h followed by Aβ ELISA (a). CHO cells were also transfected with pCMV6 APP695 or pCMV6 E1 depleted APP695, yielding cells expressing wild-type APP695 (CHO/APPwt) or truncated APP695 lacking the N-terminal E1 region (CHO/APPdE1). After 24 h, the cells were treated with 6KApoEp or ApoEp at 10 μM, or apoE3 or apoE4 at 10 μg/mL, for 3 h followed by Aβ ELISA (B, lower panel). APPwt/dE1 in cell lysates and sAPPαwt/dE1 in conditioned media derived from the transfected CHO cells were determined by WB analysis using 6E10 (B, top panels). ApoE3-, apoE4-, and ApoEp-mediated Aβ production were markedly reduced by 22C11, 6H3B5, and truncation of the dE1 region of APP, indicating that apoE-mediated Aβ production is mediated by interaction of apoE LDLR binding domain with N-terminal APP. Isotype-matched control IgG failed to reduce apoE3, apoE4, or ApoEp-mediated Aβ production (data not shown). ELISA results are representative of three independent experiments with each condition triplicated and presented as the mean (± s.d.) of Aβ (pg/mg of total intracellular protein). Asterisk indicates p < 0.05 compared with the corresponding absence of 22C11 or 6H3B5, or corresponding CHO/APPwt cells as determined by one-way ANOVA.