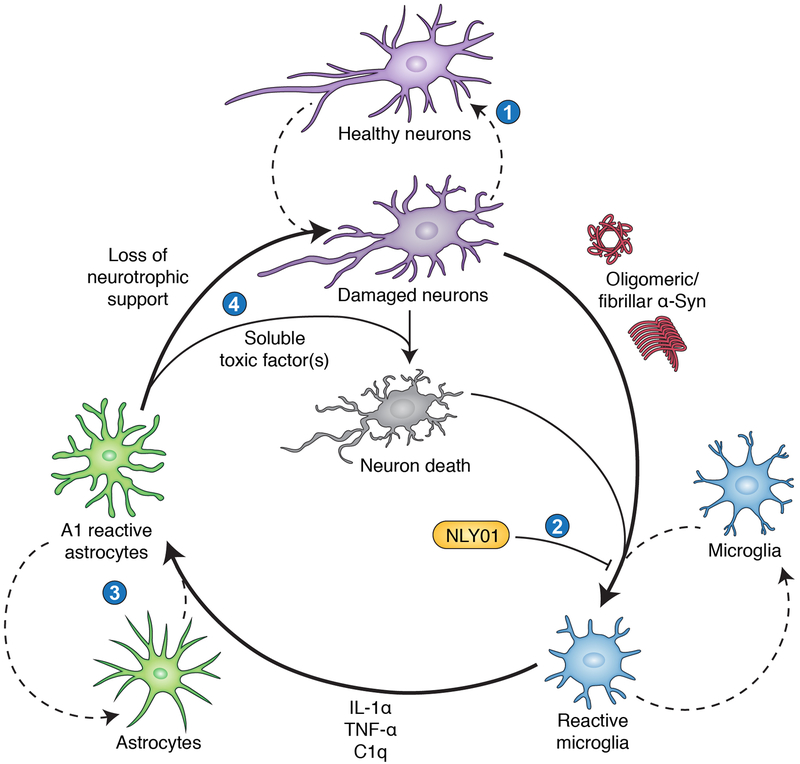
Figure 1: The neurotoxic reactive astrocyte paradigm of neurodegeneration.
A cooperative and cyclical pattern of aberrant neuron-glia interactions initiates and sustains neuronal loss in this model. We identify four key opportunities for therapeutic intervention in this cycle. (1) Aging, injury, and/or environmental or genetic influences present stresses that damage neurons via mechanisms such as dysproteostasis, mitochondrial dysfunction, oxidative/nitrative stress, lysosomal inadequacy, and DNA damage. Strategies that facilitate neuronal homeostasis or provide neurotrophic support could help reverse these processes and block progression to irreversible neuron loss. This approach may also reduce the release of immunostimulatory substances by damaged or dying neurons, including oligomeric and fibrillar α-Syn species. (2) Our work has shown that in multiple PD models, the GLP1R agonist NLY01 blocks microglial activation. This prevents subsequent transformation of astrocytes into the neurotoxic A1 reactive subtype, reducing downstream neuronal loss. (3) Future work may identify key molecular determinants of the A1 reactive astrocyte phenotype (e.g., transcriptional regulators) and targets for selectively blocking or reversing their appearance in a neuroinflammatory environment. (4) A1 astrocytes lose neurotrophic and synaptotrophic functions that normally support neuronal health, which may exacerbate disease. They also release a soluble neurotoxic factor that kills neurons. Depending on the nature of this unidentified factor, it may be possible to repress its production or release, or to render neurons resistant to its effects.