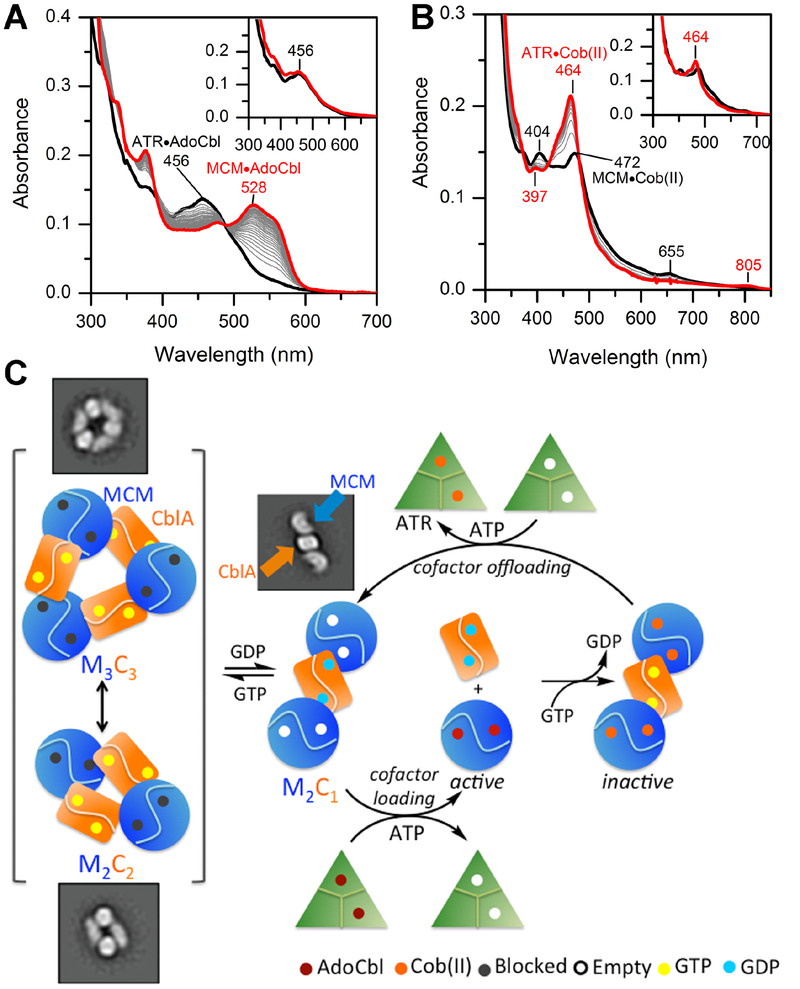
Figure 6. Model for AdoCbl loading and offloading in human.
(A) Transfer of AdoCbl (15 μM) from ATR (15 μM trimer) to MCM (15 μM) in the presence of wild-type CblA (45 μM) and 1 mM GDP or GMPPCP (inset). The initial and final spectra (30 min) are in black and red, respectively. (B) Transfer of cob(II)alamin (15 μM) bound to MCM (9 μM, black trace) in the presence of CblA (30 μM) following addition of ATR (15 μM), ATP (5 mM) and 1 mM GTP or GMPPCP (inset). The final spectrum after 15 min is in red. (C) Model for cofactor loading/off-loading highlighting a central role for the GTPase activity of CblA. Resolution of the higher order oligomers (left) to a “loading ready” M2C1 complex is driven by GTP hydrolysis, which following AdoCbl transfer separates into the individual proteins. During catalysis, occasional inactivation of MCM leads to the loss of deoxyadenosine and accumulation of cob(II)alamin, which is off-loaded in the presence of CblA in a GTPase- dependent step. For simplicity, the offloading complex is shown as M2C1 although it is currently unclear whether it can also occur from the M1C1 or the higher order complexes.