Figure 5. Interactions of Class I aaRS with the phosphate group of the 3’-terminal adenosine.
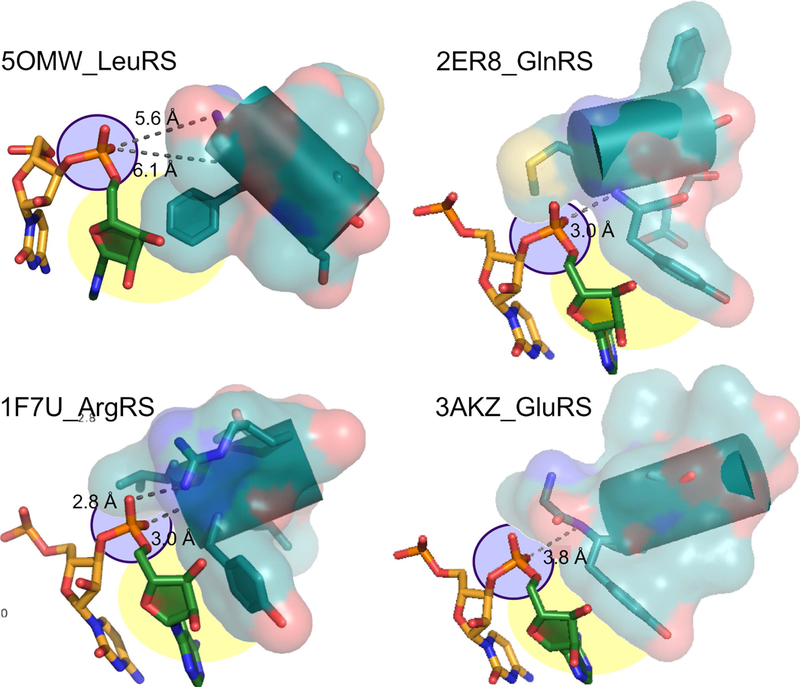
The hairpin structure of Class I isoaccepting tRNA substrates orients this phosphate group so that it points toward the N-terminus of the specificity-determining helix (purple). Hydrogen bond distances shown suggest that, except in the case of 5OMW_LeuRS, these interactions may be strong. In 1F7U_ArgRS, the interaction is reinforced by a salt bridge between the phosphate group and R350. Specificity is enhanced by the stacking interaction between the nonpolar face of the A76 ribose and a conserved aromatic sidechain immediately following the four unpaired amide nitrogens at the N-terminus of the specificity-determining helix (yellow) (adapted from (4), with permission) (adapted from (4), with permission).
