Figure 2. Stress-independent XBP1s or ATF6 activation differentially influence interactions between FTALLC and ER proteostasis factors.
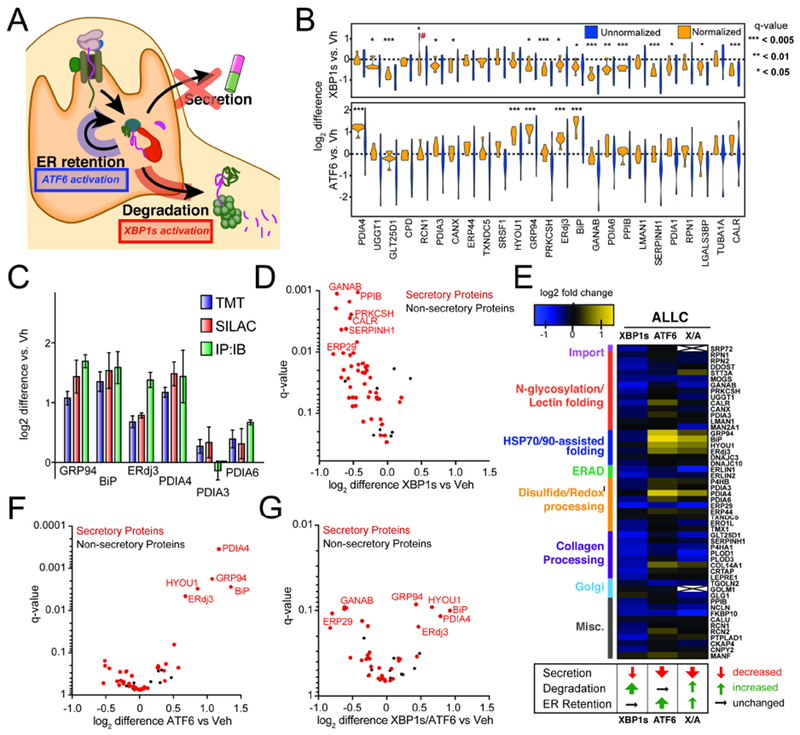
A. Illustration summarizing previous data showing how stress-independent activation of XBP1s (red) or ATF6 (blue) leads to reduced secretion of destabilized, amyloidogenic ALLC (Cooley et al., 2014). XBP1s activation modestly reduces ALLC secretion through increased targeting to degradation, while ATF6 activation significantly reduces ALLC secretion through its increased ER retention.
B. Plot showing the distribution of unnormalized (blue) and FTALLC-bait-normalized (orange) TMT interaction ratios for n = 7 biological replicates comparing the recovery of high confidence ALLC interacting proteins in anti-FLAG IPs from cells following XBP1s activation (top) or ATF6 activation (bottom). A simple normalization procedure of the protein TMT signal against the FTALLC bait protein signal across each TMT channel greatly diminishes the variance in interaction ratios. *q-value (Storey) < 0.15, **p < 0.05, ***p < 0.01; # denotes excluded outlier
C. Comparison of interaction fold changes between FTALLC and selected proteostasis factors in response to XBP1s or ATF6 activation as quantified by three independent methods: TMT-based q-AP-MS (n=7 biological replicates), SILAC-based q-AP-MS (n= 4 or 5 biological replicates), or Co-immunoprecipitation followed by quantitative immunoblotting (IP:IB; n=3–6 biological replicates).
D. Plot showing TMT interaction ratio vs. q-value (Storey) for high confidence FTALLC interacting proteins that co-purify with FTALLC in HEK293DAX cells following stress-independent XBP1s activation from n=7 biological replicates. Full data included in Supplemental Table 2.
E. Heatmap displaying the observed interactions changes between FTALLC and high confidence ER proteostasis network components following stress-independent XBP1s or ATF6 activation (data from n=7 biological replicates for ATF6 or XBP1s activation, and n = 4 for XBP1s/ATF6 co-activation). Interactors are organized by pathway or function. The previously defined impact of activating these pathways on ALLC secretion, degradation, and ER retention is shown below (Cooley et al., 2014).
F. Plot showing TMT interaction ratio vs. q-value (Storey) for high confidence FTALLC interacting proteins that co-purify with FTALLC in HEK293DAX cells following stress-independent ATF6 activation from n=7 biological replicates. Full data included in Supplemental Table 2.
G. Plot showing TMT interaction ratio vs. q-value (Storey) for high confidence FTALLC interacting proteins that co-purify with FTALLC in HEK293DAX cells following stress-independent XBP1s and ATF6 co-activation from n=4 biological replicates. Full data included in Supplemental Table 2.
