Figure 6. Overexpression of BiP partially recapitulates ATF6-dependent reductions in ALLC secretion.
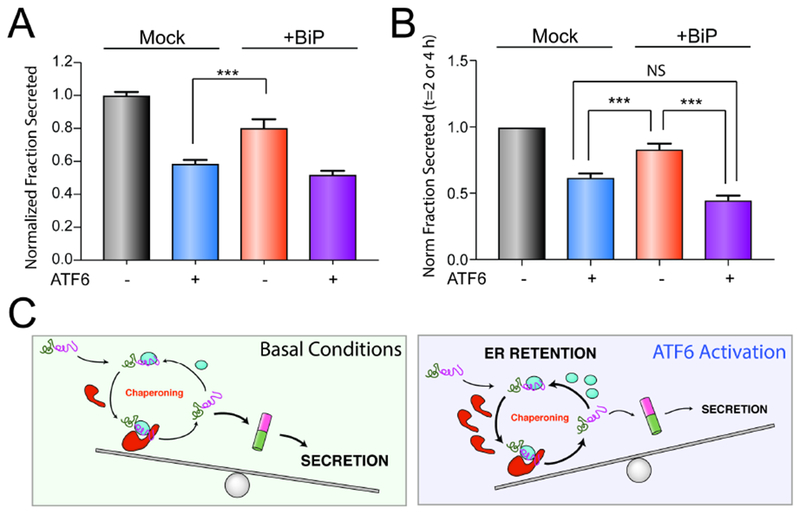
A. Graph showing the normalized fraction secretion of FTALLC in HEK293DAX cells mock-transfected or overexpressing BiP subjected to a 16 h pretreatment with vehicle or ATF6 activation, as measured by ELISA. ATF6 was activated in these cells using trimethoprim (TMP; 10 μM), as previously described (Shoulders et al., 2013). Error bars show SEM for n=6 replicates across two independent experiments. ***p<0.005 for unpaired t-test.
B. Graph showing the normalized fraction secretion of FTALLC at t = 2 or 4 h in HEK293DAX cells mock-transfected or overexpressing BiP subjected to 16 h pretreatment with vehicle or ATF6 activation, as measured by [35S] metabolic labeling. Representative autoradiograms are included in Fig. S6A. Error bars show SEM for n=4 separate measurements (2 measurements at t =2 h and 2 measurements at t=4 h) across two independent experiments. *p<0.05, ***p<0.005 for paired t-test.
C. Illustration showing a molecular model that explains the selective, ATF6-dependent reduction in destabilized ALLC secretion. The increased chaperoning environment afforded by ATF6 activation promotes iterative rounds of ALLC chaperoning to reduce ALLC folding into a trafficking competent conformation. This leads to increased ER retention of destabilized ALLC in chaperone-bound complexes that prevent its secretion to downstream secretory environments.
