Abstract
Epidemiological investigations of early childhood oral health rely upon the collection of high-quality clinical measures of health and disease. However, ascertainment of valid and accurate clinical measures presents unique challenges among young, preschool-age children. The paper presents a clinical research protocol for the conduct of oral epidemiological examinations among children, implemented in ZOE 2.0, a large-scale population-based genetic epidemiologic study of early childhood caries (ECC). The protocol has been developed for the collection of information on tooth surface-level dental caries experience and tooth-level developmental defects of the enamel in the primary dentition. Dental caries experience is recorded using visual criteria modified from the International Caries Detection and Assessment System (ICDAS), and measurement of developmental defects is based upon the modified Clarkson and O’Mullane Developmental Defects of the Enamel Index. After a dental prophylaxis (toothbrushing among all children and flossing as needed), children’s teeth are examined by trained and calibrated examiners in community locations, using portable dental equipment, compressed air, and uniform artificial light and magnification conditions. Data are entered directly onto a computer using a custom Microsoft Access-based data entry application. The ZOE 2.0 clinical protocol has been implemented successfully for the conduct of over 6000 research examinations to date, contributing phenotype data to downstream genomics and other
Keywords: dental caries, children, clinical research, protocol, caries diagnosis, early childhood caries, developmental defects of the enamel
Introduction
Epidemiological investigation of early childhood oral health relies upon the collection of high-quality clinical measures of health and disease. This, in turn, requires a clinical research protocol to ensure robust and unbiased experimental design, methodology, analysis, interpretation, and reporting of results. In the research studies of oral health domain, ascertainment of valid and accurate clinical measures presents unique challenges among young, preschool-age children. This is due to the reduced ability (e.g., limited attention span, acute anxiety, etc.) of very young children to cope with and cooperate with clinical research procedures compared to older children and adults. Nevertheless, clinical dental research is not only feasible in this young age group but also necessary to answer important biological, clinical and public health research questions [1].
Understanding the entire spectrum of social, environmental, behavioral and biological determinants of early childhood caries (ECC) [2] remains a priority. ECC is an early-onset, severe form of dental caries, that confers substantial impacts to affected children, their families, the health system, and the society in general. It is a persistent clinical and dental public health problem that is known to be influenced by social determinants of health and mediated by oral hygiene, fluoride exposure and diet [3], but also has a substantial heritable component [4]. Developmental defects of the enamel (DDE) are non-carious enamel conditions, comprising a heterogeneous group of defects of various severity and diverse etiology (i.e., hypoplastic defects of the enamel versus fluorosis). Importantly, DDE occur during dental development (i.e., prior to tooth eruption and exposure to protective and harmful exposures) and because they can increase one’s susceptibility to dental caries [5,6], their study within the context of ECC heritability can be informative.
Genetics studies of dental caries in the primary dentition caries have had mixed results [7,8] and a knowledge gap exists on the genomic basis of ECC [9]. To address this knowledge gap and add to the evidence base of social, behavioral and biological determinants of ECC, the investigators are undertaking the ZOE-G4S (Zero-out Early Childhood Caries- “Genes for Smiles”)—an NIH-funded genetic epidemiologic study conducted among a large population-based sample of preschool-age children enrolled in Head Start centers in North Carolina. The paper presents the study’s clinical protocol for the conduct of research examinations among children in the primary dentition (e.g., ages 2–5), designed to detect and record surface-level dental caries experience and tooth-level developmental defects of the enamel. The phenotype data collected using this protocol are then carried forward to genomics [15] and microbiome [16] studies, as well as other traditional clinical and epidemiologic dental investigations.
Clinical examiners (registered dental hygienists or dentists) are trained and calibrated for the dental caries examinations using a sequence of online International Caries Detection and Assessment System (ICDAS) modules, clinical photos, natural extracted teeth, and clinical examinations. Tooth-surface caries diagnoses are based on ICDAS criteria [10,11] made at the levels of health (ICDAS code: 0), early-stage (ICDAS codes: 1–2) and established/severe stage (ICDAS codes: 3–6). A dmfs (decayed, missing due to caries and filled/restored primary tooth surfaces; ranging between 0 and 88) index is created as a sum of surface-level conditions, with dmfs>0 indicating an ECC case [2], which is the primary study outcome. The presence of sealants, intra-coronal and full-coverage restorations is also recorded at the surface-level. Inter-examiner reliability in caries classification is determined by comparing un-calibrated examiners with a reference golden standard examiner. The calibration includes examination of ~25 children by all examiners, including un-calibrated ones and the gold standard, and re-examination of ~8. Examiner performance is evaluated by the weighted kappa statistic, indicating agreement with the gold standard examiner. Achieving a weighted kappa of at least 0.65, a rigorous level of inter-examiner reliability, is required for all examiners following their initial training and calibration and at annual re-evaluation sessions. The corresponding threshold for intra-examiner reliability is weighted kappa at least 0.75 [12].
Developmental defects of the enamel are measured at the tooth-level using Clarkson and O’Mullane’s modified DDE epidemiologic index [13]. Several other clinical measures are obtained as part of the research examination, including anthropometry (i.e., height, weight, BMI, BMI percentile for age and sex), extra-oral characteristics (i.e., profile, lip competence), intra-oral occlusal characteristics (i.e., molar/canine classification, overjet, overbite), dental trauma (using a modified Ellis’ index [14]) and a summary of treatment needs. With supervision and/or assistance from research staff, children’s teeth are cleaned using a toothbrush and no toothpaste; flossing is done when needed. The aim is to improve measurement and, in the process, provide instruction in dental hygiene while helping desensitize children who might be fearful of dental procedures. Before tooth cleaning, saliva and supragingival plaque samples are collected to enable downstream studies involving the human genome and oral microbiome, according to procedures detailed in [15] and [16].
2. Materials
2.1. Clinical examination equipment and instruments
Foldable dental chair (Aseptico® ADC-01P-RED, Aseptico Inc., Woodinville, WA)
Foldable hydraulic dentist stool (Aseptico® ADC-10, Aseptico Inc., Woodinville, WA)
Dental instrument tray (Aseptico® ATC-03CF, Aseptico Inc., Woodinville, WA)
Air compressor (Figure 1; custom modified TC-20 compressor, TCP Global, San Diego, CA) with air syringe and disposable tips
Examiner magnifying loupes with headlight (Orascoptic XV1™ Loupe + Light, Orascoptic, Middleton, WI)
Pediatric toothbrushes
Flossers
Disposable dental examination mirrors
US/CPTIN round-ended plastic periodontal probes with markings at 2/¾/5/7/9mm
Figure 1.
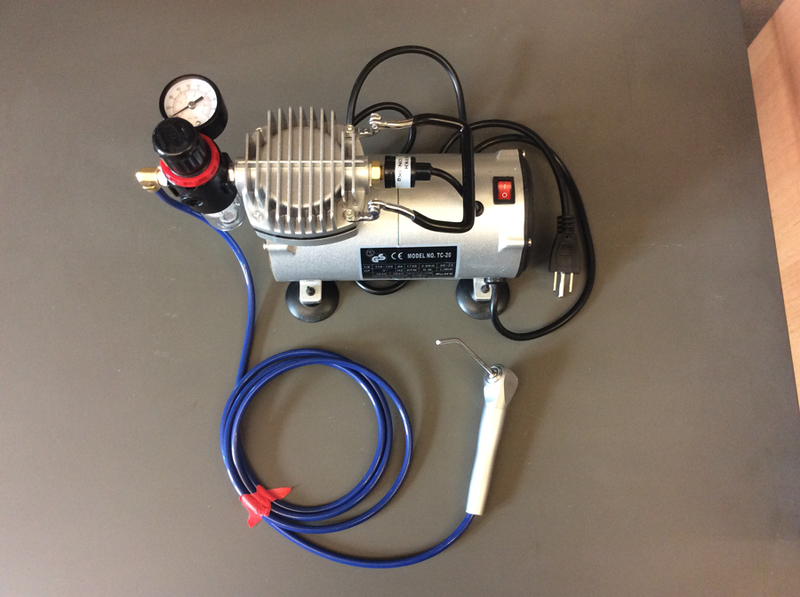
Custom modified air compressor
2.2. Clinical examination supplies
Disposable gowns
Gloves
Masks
Bibs
Hand held mirrors
Disposable plastic tips and sleeves/covers for air syringe
Barrier tape
Plastic chair and tray covers
Surface disinfecting wipes
Hand sanitizer
2×2 gauze
Paper towels
Sterile pouches for used probes
2.3. Anthropometric measurement equipment and supplies
Portable digital stadiometer (seca® 213 or seca® 264, seca GmbH & Co. KG, Hamburg Germany)
Portable digital scale (DS6150, Doran® Remote Indicator Scale, Doran, Batavia, IL)
Carrying case for portable digital stadiometer and scale (Seca® 414, seca GmbH & Co. KG, Hamburg Germany
iPad tablet with ‘ChildBMI’ app (Tactio Health Group) for pediatric BMI percentile calculation
Printed “subject cards” for intermediate recording of height, weight, BMI and BMI percentile values prior to electronic data capture
2.4. Electronic data recording and other study source documents
Laptop
Microsoft Access-based, custom-written data entry application (DEA)
Handheld barcode reader (for scanning of barcode labels with participant IDs)
Printed screenshots of DEA data entry screen (as back-up)
External monitor for examiner
Power strips, extension cords, and adapters
Blue masking (floor) tape
Encrypted, portable USB storage device (e.g., IronKey™, Kingston Technology Company, Inc., Fountain Valley, CA)
2.5. Biospecimen collection
3. Methods
Research examination teams use the mobile dental equipment, instruments, and supplies to set up clinical examination stations (Figure 2) where there is suitable space and a power outlet in community locations (e.g., classrooms). Each examiner has custom-fit magnifying loupes with headlights at their disposal (§2.1) and is trained to use basic child behavior guidance and management techniques routinely used in pediatric dentistry, including tell-show-do (TSD), positive reinforcement, negative reinforcement, voice control, modeling and labeling. Children whose legal guardians have provided informed consent are examined -at least 30, preferably 60 minutes- after they have had breakfast or snack. The families (and teachers, where applicable) are instructed not to brush the children’s teeth the morning before the examination--the examiner cleans the teeth with a toothbrush (no toothpaste) and floss (when needed) after biospecimen collection, and the teeth are dried before the examination. The examiner completes the exam and all data are directly recorded electronically by a designated recorder using the DEA. The procedures are carried out as follows:
Figure 2.

Example clinical research set up in a non-clinical setting
3.1. Pre-clinical procedures and assessments
Greet the child addressing them by their name and ideally kneeling down to their eye level
Explain the procedures that will follow in an age-appropriate manner
Offer stickers at each examination ‘station’ beginning at initial contact
Record height, weight, and compute BMI and BMI percentile for age and sex.
Collect saliva sample with OG-575 kit. This can be accomplished by the child sitting upright in any (regular, or dental chair).
Collect supragingival plaque samples using the sterile toothpicks in a semi-reclined position, ideally a dental chair. One sample (plaque sample A) from the upper right dentition (facial surfaces of #A, #B, #C, #D and #E according to the Universal nomenclature system; or #55, #54, #53, #52 and #51 according to the FDI system) and another sample (plaque sample B) from the upper left dentition (facial surfaces of #F, #G, #H, #I and #J, or #61, #61, #63, #64 and #61).
Store plaque A is stored in an RNAlater tube and plaque B in a CryoVial to freeze immediately, until transfer to a lab and storage in −80oC.
3.2. Clinical procedures and assessments-dental caries and DDE
Clean teeth with a toothbrush and provide oral hygiene instruction for all children. Clean inter-proximal areas with dental floss as needed.
Record extra-oral characteristics as follows: profile (1=straight, 2= convex, 3=concave; 9=unable to assess); lip competence (1=competent: <3mm distance between upper and lower lip and no mentalis muscle strain; 2= incompetent: ≥3mm distance between upper and lower lip or mentalis muscle strain; 9=unable to assess).
Record intra-oral characteristics as follows: overjet (mm; 999=unable to assess); overbite (percent coverage of lower incisors from the upper incisors; 999=unable to assess); right/left molar anterior-posterior classification (1=flush, 2=mesial step, 3=distal step, 9=unable to assess); right/left canine anterior-posterior classification (1=class I, 2=class II, 3=class III, 9=unable to assess).
Dry each tooth to be examined for surface-level dental caries lesions or other conditions with compressed air (or alternatively with a 2×2 gauze) and visualize with artificial light, magnification and disposable mirror. Recording is based on a two-step system: first provide a tooth-level code (Table 1) and then, as indicated, 4 or 5 surface-level codes (Table 2). The purpose of this system is to a) expedite data collection in cases where all surfaces within a tooth receive the same diagnosis (i.e., healthy, crowned, extracted due to caries, missing due to trauma, permanent tooth is present, unable to assess) and b) allow the implementation of logical checks, thus minimizing data entry errors. Five surfaces (occlusal, facial, lingual, mesial and distal) are assessed for molar teeth and four surfaces (facial, lingual, mesial and distal) for anterior teeth.
Examination for DDE is best done on non-desiccated teeth and for this reason the child is asked to close their mouth and swallow or lick their teeth after the dental caries examination. Examine only the facial/buccal surface of all teeth for non-carious enamel defects that are of ≥1mm diameter, using the type and extent codes listed in Table 3. If more than one condition is present (e.g. diffuse opacity and hypoplasia) record the extent of the largest one. If more than two-thirds of a tooth’s facial/buccal surface is heavily restored, badly decayed or fractured, then do not assess it for DDE (i.e., 9=Unable to assess).
Table 1.
Tooth-level diagnostic and classification codes for recording of dental caries
| Code | Explanation |
|---|---|
| 1 | Sound, no decay or restorations2 |
| 2 | Sealed1 |
| 3 | Decayed or filled (restored)1 |
| 4 | Crown (stainless steel or other)2 |
| 5 | Missing due to caries2 |
| 6 | Exfoliated2 |
| 7 | Missing due to trauma2 |
| 8 | Permanent tooth present2 |
| 9 | Unable to score2 |
Tooth surface-level codes are entered by the examiner only for tooth-level codes 2 and 3. See Table 2.
For the other tooth-level codes, tooth-surface codes are auto-filled by the DEA, as follows: Tooth-level code 1—Sound, auto-filled surface-level codes: 0; Tooth-level code 4—Crown, auto-filled surface-level codes: 44; Tooth-level code 5—Missing due to caries, auto-filled surface-level codes: 55; Tooth-level codes 6,7,8,9—Exfoliated, Missing due to trauma, permanent tooth present, unable to score, auto-filled surface-level codes: 99.
Table 2.
Surface-level diagnostic and classification codes for recording of dental caries
| Code | Explanation |
|---|---|
| 0 | Sound, no caries lesion (ICDAS: 0) |
| 50 | Restored, no caries lesion |
| 80 | Sealed, no caries lesion1 |
| Caries | |
| 10 | Arrested enamel lesion (enamel-only)2 |
| 11 | Early stage caries lesion (ICDAS: 1–2) |
| 12 | Established/severe caries lesion (ICDAS: 3–6) |
| Restored and separate caries lesion | |
| 30 | Restored and separate arrested enamel lesion (enamel-only)2 |
| 31 | Restored and separate early stage caries lesion (ICDAS: 1–2) |
| 32 | Restored and separate established/severe caries lesion (ICDAS: 3–6) |
| Restored and recurrent caries lesion | |
| 40 | Restored and recurrent arrested enamel lesion (enamel-only)2 |
| 41 | Restored and recurrent early stage caries lesion (ICDAS: 1–2) |
| 42 | Restored and recurrent established/severe caries lesion (ICDAS: 3–6) |
| Sealed and separate caries lesion | |
| 60 | Sealed and separate arrested enamel lesion (enamel-only)2 |
| 61 | Sealed and separate early stage caries lesion (ICDAS: 1–2) |
| 62 | Sealed and separate established/severe caries lesion (ICDAS: 3–6) |
| Sealed and recurrent caries lesion | |
| 70 | Sealed and recurrent arrested enamel lesion (enamel-only)2 |
| 71 | Sealed and recurrent early stage caries lesion (ICDAS: 1–2) |
| 72 | Sealed and recurrent established/severe caries lesion (ICDAS: 3–6) |
| Auto-filled codes | |
| 44 | Surface of tooth with a crown, i.e., if tooth code=4 |
| 55 | Surface of tooth that has been extracted due to caries, i.e., if tooth code=5 |
| 99 | Surface of tooth that has exfoliated, is missing due to trauma, a permanent tooth is present, or is unable to score for any other reason, i.e., if tooth code=6, 7, 8, or 9 |
Any evidence of sealant material present (i.e., partially retained) is recorded as sealant
Caries lesion activity is only considered for enamel-level non-cavitated (i.e., white spot) lesions
Table 3.
Diagnostic and classification codes for recording of type and extent of developmental defects of the enamel
| Type | |
|---|---|
| Code | Explanation |
| 0 | Normal (no DDE) |
| 1 | Demarcated opacity |
| 2 | Diffused opacity |
| 3 | Hypoplasia |
| 4 | Demarcated opacity + diffused opacity |
| 5 | Demarcated opacity + hypoplasia |
| 6 | Diffused opacity + hypoplasia |
| 7 | Demarcated opacity + diffused opacity + hypoplasia |
| 8 | Other defect |
| 9 | Unable to score |
| Extent | |
| 0 | Normal (no DDE) |
| 1 | < 1/3 of facial/buccal tooth surface |
| 2 | At least 1/3 but less than 2/3 of facial/buccal tooth surface |
| 3 | At least 2/3 of facial/buccal tooth surface |
| 9 | Unable to score |
3.3. Measurement of other clinical characteristics: trauma, treatment needs, behavior, completion and examination notes
Examine the 4 primary maxillary incisors for evidence of dental trauma using the modified Ellis [14] classification criteria listed in Table 4.
Provide a behavior assessment score at the end of the examination to provide a global measure of the child’s cooperation using the Frankl behavior scale [17], as follows: 4=completely cooperative, enjoys the process, 3=cooperative, somewhat reluctant/shy, 2=uncooperative, very reluctant/shy, 1=completely uncooperative. In cases where part or the entirety of the research examination was not done due to non-cooperation, a score of 1 is given.
Provide a ‘treatment needs’ score, according to the level and urgency of dental treatment that the child requires, as follows: 1=no obvious problems were found today; establish or maintain a dental home and continue routine dental visits, 2=possible problems were found today; please check at the next dental visit, 3=problems were found today that require treatment as soon as possible.
Provide a ‘completion’ code indicating whether all planned research procedures were completed at this visit: 1=completed; 2=not complete. A built-in check routine in the DEA will prevent entering ‘1’ if a data field is incomplete.
Provide free-text comments in the appropriate comments box, if and as needed. In this section, general remarks protocol deviations (e.g., changes in procedures due to behavior/cooperation) and any extraordinary findings can be recorded. A comment is mandated if a partial exam (i.e., ‘not complete’ code) exam was conducted.
Table 4.
Diagnostic and classification codes for recording of dental trauma
| Code | Explanation |
|---|---|
| 0 | No evidence of trauma |
| 1 | Simple crown fracture, involving little or no dentin |
| 2 | Extensive crown fracture, involving considerable dentin surface, without pulp exposure |
| 3 | Extensive crown fracture, involving considerable dentin surface, with pulp exposure |
| 4 | Displacement of the tooth due to trauma |
| 5 | Necrotic/discolored tooth (due to trauma) w/wo crown fracture |
| 6 | Total tooth loss due to trauma |
| 7 | Unable to assess |
Table 5.
Examples of 10 person-level caries traits (disease indices and definitions) that can be constructed using the surface-level caries diagnosis codes used in this protocol
| Trait 1 | Trait 2 | Trait 3 | Trait 4 | Trait 5 | Trait 6 | Trait 73 | Trait 83 | Trait 93 | Trait 103 | surface codes1 | description |
|---|---|---|---|---|---|---|---|---|---|---|---|
| composite index | two-level disease | ECC definition |
established/ severe disease |
caries experience, two-level |
caries experience, established/ severe | “restored” disease |
“unrestored” disease |
“healthy”, sealed |
“healthy”, non-sealed |
||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | Sound |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 80 | Sealant |
| 2 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 10, 11 60, 61 70, 71 |
Caries: early stage (arrested or active) |
| 2 | 1 | 1 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 302, 312 402, 412 |
Recurrent or Secondary Caries: early stage (arrested or active) |
| 3 | 2 | 1 | 1 | 2 | 1 | 0 | 1 | 0 | 0 | 12 ,322, 422, 62, 72 | Caries: established/severe |
| 4 | 2 | 1 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 50 | Restored |
| 5 | 2 | 1 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 44 [auto-filled] | Crowned |
| 6 | 2 | 1 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 55 [auto-filled] | Missing due to caries |
Surfaces with code: 99 (when tooth code is 6,7,8 or 9) are excluded
These surfaces have been restored, and thus can be grouped with trait 4 if the interest is examining ‘disease severity’; if the interest is in active disease, then these surfaces can be counted according to the lesion classification, i.e., as early stage (trait 2) or established/severe (trait 3)
For traits 7–10 disease is considered at the established/severe level
Additional person-level indicator variable definitions: restorativetx=1 if one or more surfaces are in (30, 31, 32, 40, 41, 42, 50, 44, 55), else restorativetx=0; sealantstx=1 if one ore more surfaces are in (80, 60, 61, 62, 70, 71, 72), else sealantstx=0
Acknowledgement
This work was supported by a grant from the National Institutes of Health, National Institute of Dental and Craniofacial Research, U01-DE025046.
Footnotes
Notes
Explaining all procedures to the participating children using the “tell-show-do” technique and actively involving them in the process, where they can even hold a hand mirror to watch and for example, ‘help the examiner count teeth’, are very helpful strategies—especially in cases of reluctant or anxious children.
In cases of uncertainty about the diagnosis/classification of a condition prefer ‘under-calling’ it. For instance, if uncertain whether an early stage (incipient) caries lesion is present or not, prefer to record the lesser diagnosis, which -in this case- is health (e.g., surface-code 0). Apart from resulting in a more conservative estimation of disease burden, this rule is helpful in training and calibration, so that examiners have a uniform rule for resolving uncertainties.
Multiple traits besides the ECC case status (binary) and the dmfs index (0–88) can be constructed using the diagnostic codes collected as part of the protocol. The construction of 10 example traits and two treatment indicator variables is presented in Table 5. For example, trait 3 is the “classic” ECC definition and trait 4 is the same case status definition not considering early-stage lesions.
The custom modified air compressor referred to in §2.1 has proven to be an excellent solution for the provision of compressed air during the visual dental caries examinations in the field—it is lightweight, silent and produces sufficient airflow.
Consider a tooth present if any part of it has penetrated the mucosa and record any condition that can be detected on its erupted portion.
Overjet measurements can be performed if any pair of upper-lower primary central incisors is present (e.g., #F-O or #E-P).
Placing multiple layers of plastic covering on dental chair and tray saves time when turning over the set up for the next participant.
References
- 1.Casamassimo PS, Lee JY, Marazita ML, Milgrom P, Chi DL, Divaris K. Improving children’s oral health: an interdisciplinary research framework. J Dent Res 2014. October;93(10):938–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drury TF, Horowitz AM, Ismail AI, Maertens MP, Rozier RG, Selwitz RH. Diagnosing and reporting early childhood caries for research purposes. A report of a workshop sponsored by the National Institute of Dental and Craniofacial Research, the Health Resources and Services Administration, and the Health Care Financing Administration. J Public Health Dent 1999. Summer;59(3):192–197. [DOI] [PubMed] [Google Scholar]
- 3.Divaris K Predicting Dental Caries Outcomes in Children: A “Risky” Concept. J Dent Res 2016. March; 95(3):248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballantine J, Carlson JC, Zandona A, Agler CS, Zeldin LP, Rozier G, Roberts MW, Basta PV, Luo J, Antonio-Obese ME, McNeil D, Weyant R, Crout RJ, Slayton R, Levy S, Shaffer JR, Marazita ML, North KE, Divaris K. Exploring the genomic basis of early childhood caries: a pilot study. Int J Paed Dentistry doi: 10.1111/ipd.12344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seow WK. Developmental defects of enamel and dentine: challenges for basic science research and clinical management. Aust Dent J 2014. June;59 Suppl 1:143–54. [DOI] [PubMed] [Google Scholar]
- 6.Caufield PW, Li Y, Bromage TG. Hypoplasia-associated severe early childhood caries--a proposed definition. J Dent Res 2012. June;91(6):544–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vieira AR, Modesto A, Marazita M. Caries: Review of Human Genetic Research. Caries Res 2014; 48:491–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaffer JR, Wang X, Feingold E, et al. Genome-wide association scan for childhood caries implicates novel genes. J Dent Res 2011; December; 90(12):1457–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Divaris K Precision Dentistry in Early Childhood: The Central Role of Genomics. Dent Clin North Am 2017. July; 61(3):619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitts N “ICDAS”--an international system for caries detection and assessment being developed to facilitate caries epidemiology, research and appropriate clinical management. Community Dent Health 2004. September;21(3):193–8. [PubMed] [Google Scholar]
- 11.Ismail AI, Sohn W, Tellez M, Amaya A, Sen A, Hasson H, Pitts NB. The International Caries Detection and Assessment System (ICDAS): an integrated system for measuring dental caries. Community Dent Oral Epidemiol 2007. June;35(3):170–8. [DOI] [PubMed] [Google Scholar]
- 12.Banting DW, Amaechi BT, Bader JD, Blanchard P, Gilbert GH, Gullion CM, Holland JC, Makhija SK, Papas A, Ritter AV, Singh ML, Vollmer WM. Examiner training and reliability in two randomized clinical trials of adult dental caries. J Public Health Dent 2011. Fall;71(4):335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarkson J, O’Mullane D. A modified DDE Index for use in epidemiological studies of enamel defects. J Dent Res 1989. March;68(3):445–50. [DOI] [PubMed] [Google Scholar]
- 14.Ellis RG, Davey KW. The classification and treatment of injuries to the teeth of children: a reference manual for the dental student and the general practitioner Year Book Publishers Incorporated, Chicago: 1970. [Google Scholar]
- 15.Agler AS, Shungin D, Ferreira Zandoná AG, Basta PV, Luo J, Cantrell J, Pahel TD Jr., Meyer BD, Shaffer JR, Schäefer AS, North KE, Divaris K Protocols, methods and tools for genome-wide association studies (GWAS) of dental traits. Methods Mol Biol In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shungin D, Basta PV, Azcarate-Peril AM, Ferreira Zandoná AG, Cho H, Wu D, Ginnis J, Cantrell J, Pahel TD Jr., Roach J, Divaris K Protocol for clinical, laboratory and bioinformatics pipelines for oral microbiome studies of dental caries involving metagenomics, metatrascriptomics and metabolomics. Methods Mol Biol In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frankl SN, Shiere FR, Fogels HR. Should the parent remain with the child in the dental operatory? J Dent Child 1962;29:150–163. [Google Scholar]


