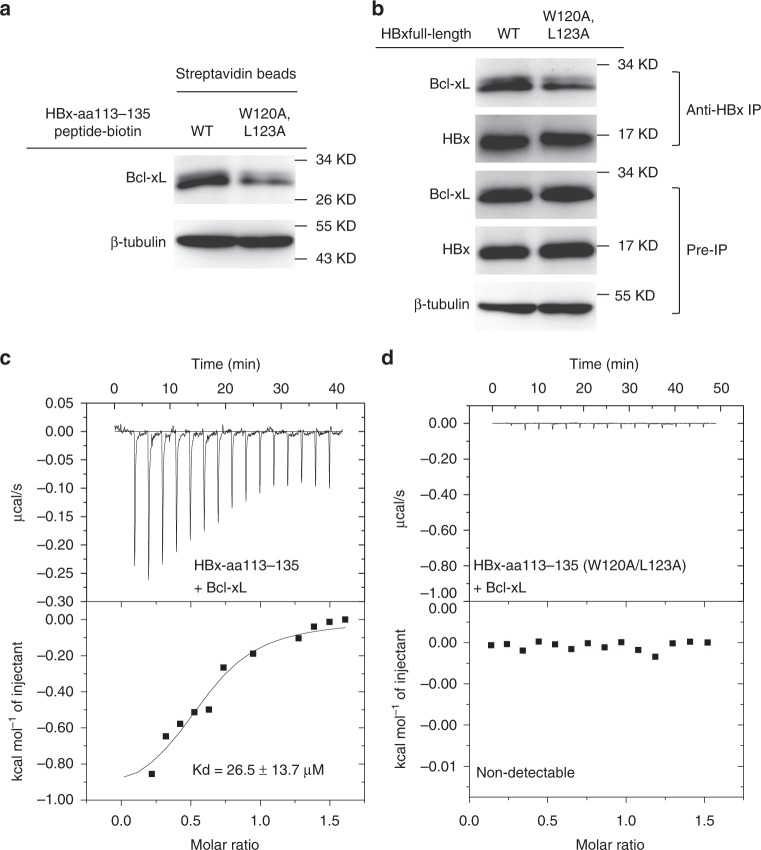
Fig. 2.
HBx binds endogenous Bcl-xL through the BH3-like domain. a HBx-aa113–135 interacts with Bcl-xL in HepG2 cell lysate. Co-precipitation experiments were performed in the HepG2 cell lysate using biotinylated HBx-aa113–135 peptides (wild type (WT)) and corresponding mutant peptides (W120A/L123A), respectively. The results were analyzed by immunoblotting (IB) using anti-Bcl-xL antibody. β-Tubulin was measured and analyzed as an input control. b Full-length HBx interacts with Bcl-xL in HepG2 cells. Co-immunoprecipitation (co-IP) experiments were performed in HepG2 cells transfected with pTT22m-HBx-WT or pTT22m-HBx(W120A/L123A). The cell lysate was precipitated by an anti-HBx antibody and analyzed by immunoblotting using an anti-Bcl-xL antibody. c Isothermal titration calorimetry (ITC) result shows the dissociation constant between HBx113-135 and Bcl-xL is 26.5 ± 13.7 μM. d The interaction between HBx-aa113–135(W120A/L123A) and Bcl-xL is non-detectable by ITC. Source data are provided as a Source Data file