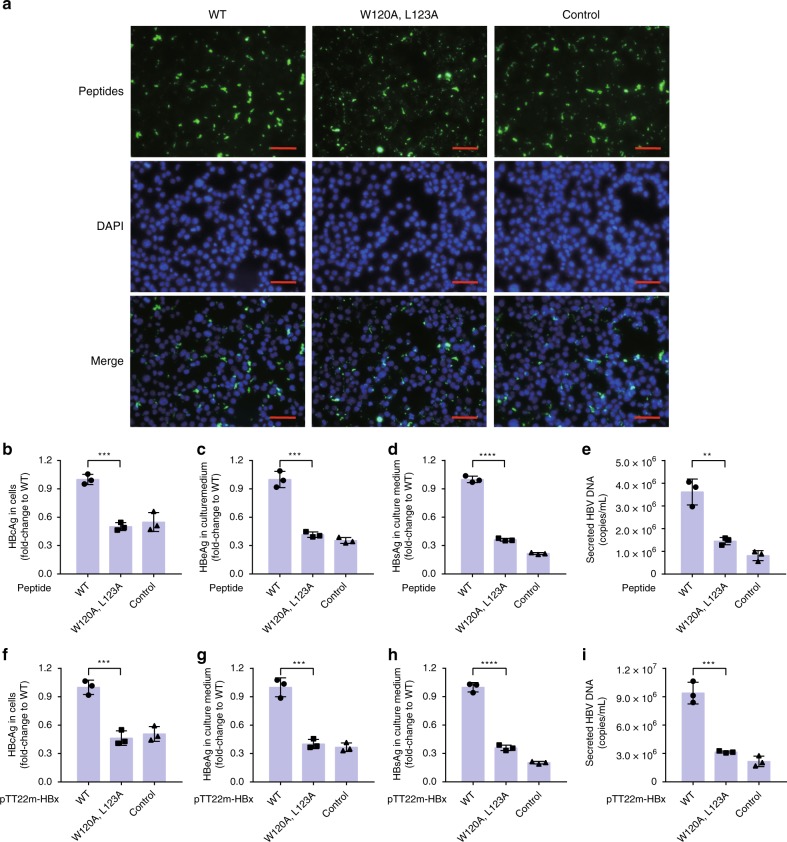
Fig. 3.
The HBx-BH3-like domain is critical for HBV production in HepG2 cells. a–e C-terminal biotinylated HBx-aa113–135 peptides (both wild-type (WT) and the W120A/L123A double mutant) were delivered into HepG2 cells transfected with pHBV1.3-Xnull using PEP-1 nanoparticles (Methods) and analyzed by streptavidin-FITC (fluorescein isothiocyanate) for transfection efficiency (a). Scale bar is 50 μm. An unrelated 23-aa peptide was used as a control (Control). The intracellular expression levels of HBV core antigen (HBcAg (b) and the levels of HBV e-antigen (HBeAg) (c), HBV S-antigen (HBsAg) (d), and HBV DNA (e) in the culture medium collected from HepG2 cells were measured (Methods). The results are shown as fold change to the WT HBx-aa113–135 peptide (WT). f–i) HepG2 cells were co-transfected with pHBV1.3-Xnull and pTT22m-HBx-WT (WT), pHBV1.3-Xnull and pTT22m-HBx(W120A/L123A), or pHBV1.3-Xnull and the pTT22m vector control (Control). Intracellular expression levels of HBcAg (f) and the levels of HBeAg (g), HBsAg (h), and HBV DNA (i) in the culture medium from HepG2 cells were measured similarly. The results are shown as fold change to the combination of pHBV1.3-Xnull and pTT22m-HBx-WT (WT). Data shown represent mean ± SD. Significance is indicated on the top. **P < 0.01, ***P < 0.001, ****P < 0.0001; two-tailed unpaired t tests (n = 3 biologically independent samples). Source data are provided as a Source Data file