Fig. 5.
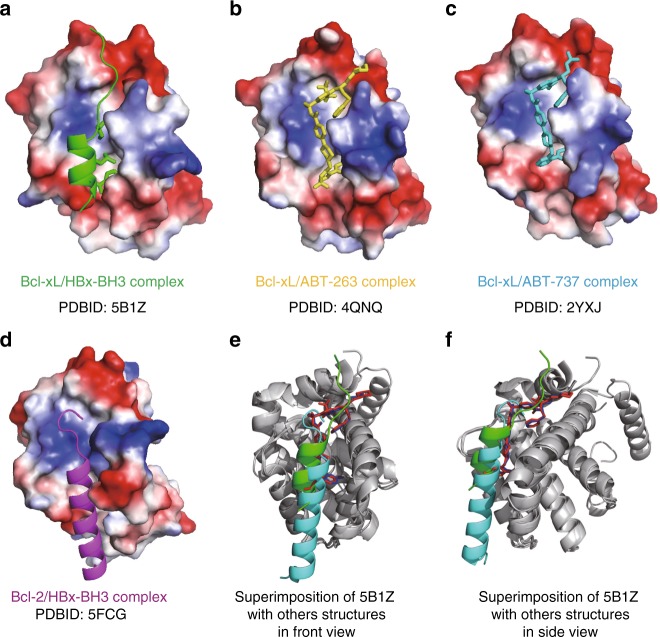
Comparison of Bcl-xL/HBx-aa113–135, Bcl-xL/ABT-263, Bcl-xL/ABT-737, and Bcl-2/HBx-aa110–135 structures. a Cartoon structure of Bcl-xL in complex with HBx-aa113–135 (PDB ID 5B1Z). b Cartoon structure of Bcl-xL in complex with ABT-263 (PDB ID 4QNQ). c Cartoon structure of Bcl-2 in complex with ABT-737 (PDB ID 2YXJ23). d Cartoon structure of Bcl-2 in complex with HBx-aa110–135 (PDB ID 5FCG8). The structures of Bcl-xL and Bcl-2 are in surface mode, the HBx-BH3-like motifs are in cartoon mode, and key residues and ABT molecules are indicated in stick mode. Notably, compared with the Bcl-xL/HBx-aa113–135 structure, the HBx-BH3-like helix in the Bcl-2/HBx-aa110–135 structure is shifted ~8 Å towards the N-terminus. Hence, the side chain of the crucial Trp120 residue is pointing to the opposite direction and more than 10 Å away from the conserved hydrophobic pocket formed by the side chains of Phe105, Leu108, Val126, and Phe146 that is critical for binding the HBx-BH3-like motif. e, f The structural superimposition (front and side views) of Bcl-xL/HBx-BH3-like complex, Bcl-xL/ABT-263 complex, Bcl-xL/ABT-737 complex, and Bcl-2/HBx-BH3-like complex. Bcl-xL and Bcl-2 are colored in dark gray. The HBx-BH3-like peptide in the Bcl-xL/HBx-BH3-like complex, ABT-263, ABT-737, and the HBx-BH3-like peptide in the Bcl-2/HBx-BH3-like complex are colored in green, red, blue, and cyan, respectively