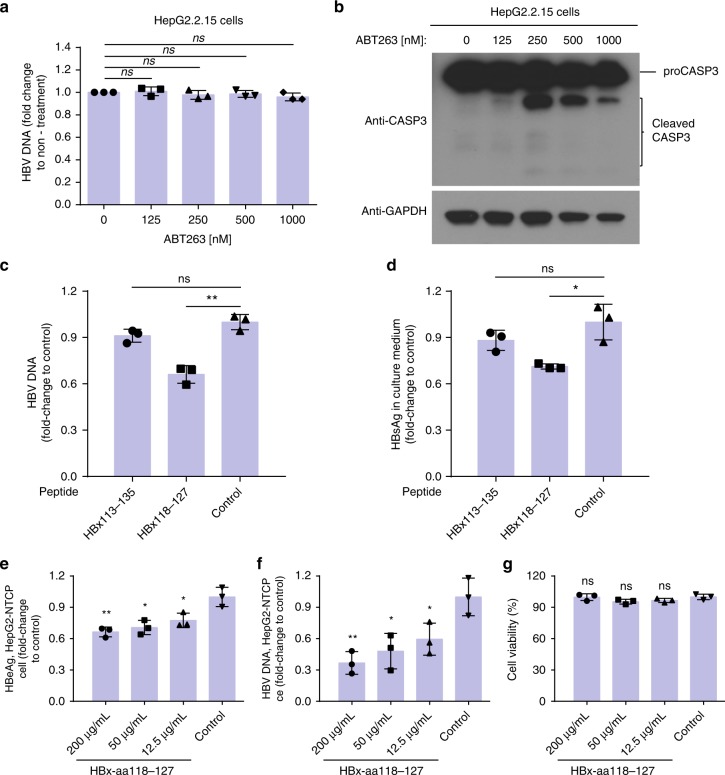
Fig. 6.
The HBx-aa118–127 peptide, but not ABT-263, inhibits HBV replication and transcription in HepG2.2.15 cells. a HepG2.2.15 cells were treated with 125, 250, 500, and 1000 nM of ABT-263 for 2 days. Cytoplasmic HBV viral particles were isolated and the viral DNA replication intermediates were quantified (Methods). The results represent the fold change of the replicative intermediates in HepG2.2.15 cells from the indicated treatments, compared with those without treatment. b HepG2.2.15 cells were treated with the indicated concentrations of ABT-263 for 2 days. The lysate was analyzed by immunoblotting using antibodies to caspase-3 (CASP3) and GAPDH (glyceraldehyde 3-phosphate dehydrogenase). c, d The HBx-aa113–135, HBx-aa118–127, or the control peptides were delivered into HepG2.2.15 cells using PEP-1 nanoparticles (Methods). The levels of HBV DNA (c) and the levels of HBV S-antigen (HBsAg) expression (d) in culture medium collected from HepG2.2.15 cells were measured (Methods). The results are shown as fold change compared to that of the control peptide. e–g HepG2-NTCP cells were transfected with HBx118–127 peptide with concentration of 12.5, 12.5, and 50 μg/ml or unrelated control peptide during HBV infection (multiplicity of infection (MOI) = 200), respectively. e HBV e-antigen (HBeAg), f HBV DNA, and g cell viability were measured at day 8 after HBV infection. Data shown represent mean ± SD. Significance is indicated on the top. Not significant (ns): *P < 0.05, **P < 0.01; two-tailed unpaired t tests (n = 3 biologically independent samples). Source data are provided as a Source Data file