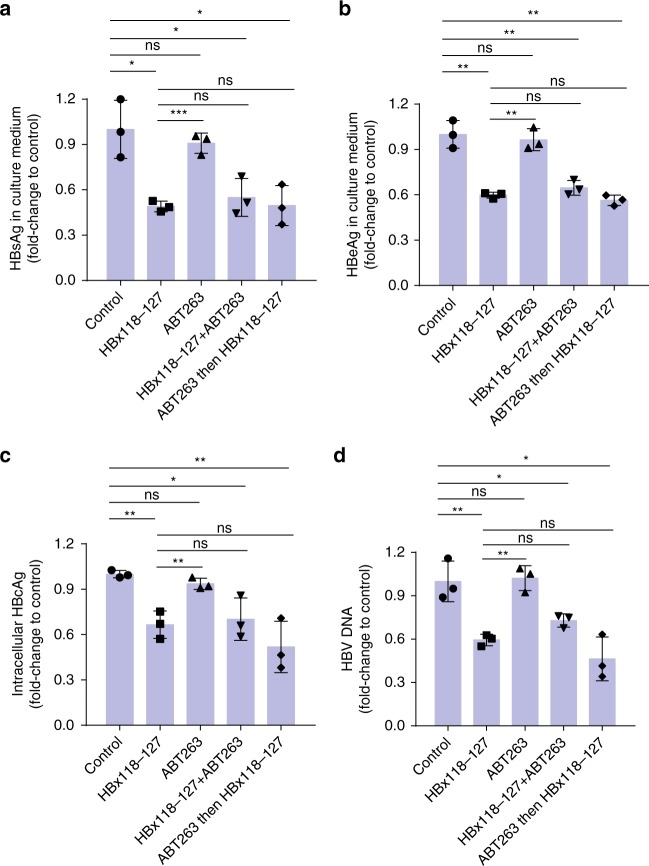
Fig. 7.
ABT-263 does not affect the inhibitory effect of HBx118–127 on HBV replication in HepG2.2.15 cells. a–d HepG2.2.15 cells were pre-seeded 12 h before peptide treatment. In all panels, from left to right (bars 1–5), cells were treated with placebo buffer, HBx118–127 only, ABT-263 only, HBx118–127 and ABT-263 together, and ABT-263, followed by HBx118–127, respectively. The cells in bars 1–4 were treated for 48 h, whereas the cells in bar 5 were first treated by ABT-263 for 12 h, followed by treatment with HBx118–127 for another 36 h. The final concentration of ABT-263 was 1 μM in the culture medium, while HBx-aa118–127 was delivered by PEP-1 nanoparticles. After 48-h treatment, the protein expression levels of HBV S-antigen (HBsAg) (a) and HBV e-antigen (HBeAg) (b) in the culture medium, the intracellular protein expression level of HBV core antigen (HBcAg) (c), and the HBV viral DNA tilter (d) in the culture medium of HepG2.2.15 cells were measured (Methods). The results are shown as fold changes compared to the values obtained from the cells treated with the placebo buffer. Data shown represent mean ± SD. Significance is indicated on the top. Not significant (ns): *P < 0.05, **P < 0.01, ***P < 0.001; two-tailed unpaired t tests (n = 3 biologically independent samples). Source data are provided as a Source Data file