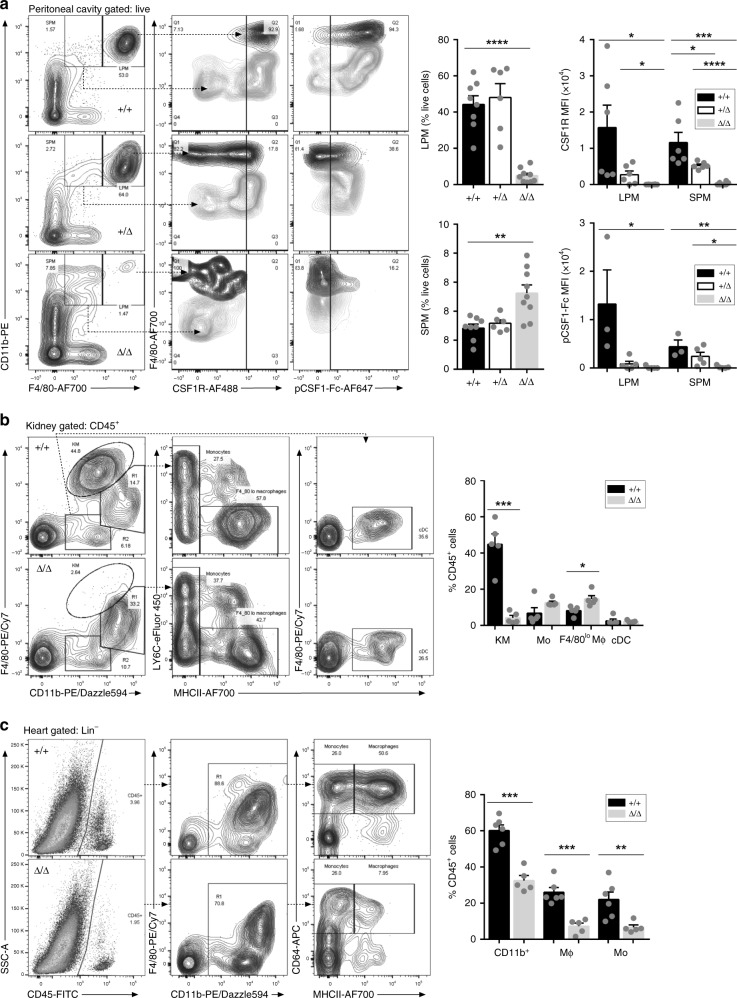
Fig. 5.
FIRE deletion results in loss of macrophages in the peritoneal cavity, kidney, and heart. a Flow cytometry profiles show peritoneal cells from representative Csf1r+/+ and Csf1rΔFIRE/ΔFIRE mice using the gating strategy described by Bain et al.36. Small peritoneal macrophages (SPM) were identified as F4/80loCD11b+ and large peritoneal macrophages (LPM) as F4/80hiCD11b+ (Panel 1). These populations were analyzed for CSF1R expression (Panel 2) and binding of pCSF1-Fc (Panel 3). n = 8 +/+, 6+/Δ and 9 Δ/Δ mice aged between 10 and 15 weeks, from five experiments. P = 0.012–0.048 (*), 0.002–0.006 (**), 0.001 (***) and <0.0001 (****). b Leukocyte populations were isolated from enzymatically digested kidneys and gated for CD45 expression. The flow cytometry profiles show analysis of representative Csf1r+/+ and Csf1rΔFIRE/ΔFIRE mice. Kidney macrophages (KM) were identified as F4/80hiCD11blo (Panel 1), pooled LY6C−/+ monocytes (Mo) as F4/80loMHCII− (Panel 2), putative monocyte-derived macrophages as F4/80loMHCII+ (Panel 2), and conventional dendritic cells (cDC) as F4/80−CD11bloMHCII+ (Panel 3). n = 5 mice per genotype aged 8–10 weeks, from three experiments. P = 0.015 (*) and 0.0001 (***). c Single cell suspensions of enzymatically digested hearts from the same cohort were analyzed by flow cytometry. Cells were gated lineage− (Lin− = CD3/CD19/LY6G). n = 6+/+ and 5 Δ/Δ from three experiments. MΦ macrophage, Mo monocyte. P = 0.009 (**), 0.0003 (*** MΦ), and 0.0001 (*** CD11b+). All source data are provided within a Source Data excel file. Graphs show mean + SEM and P values were determined by two-tailed t-tests