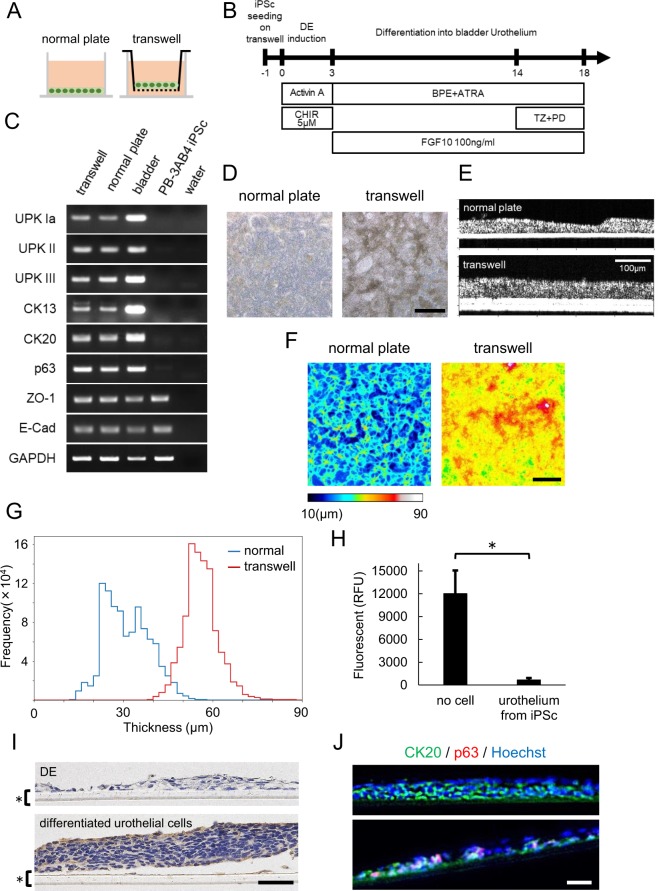
Figure 6.
Transwell culture generated more stratified epithelium than normal plate culture. (A) Schematic images of normal plate culture (left) and transwell culture (right) for urothelial differentiation. (B) An overview of the culture protocol on transwell. (C) A semi-quantitative RT-PCR analysis of urothelial markers (UPK Ia, UPK II, UPK III), transitional differentiation markers (CK13, CK20) and other differentiation markers (p63, ZO-1 and E-Cadherin) in differentiated cells cultured in the normal plates and transwells. Total RNA of human bladder tissue was used as a positive control. Full-length gels are presented in Supplementary Figure 7. (D) Phase contrast images of differentiated cells at day 18 on normal plate (left panel) and transwell (right panel). Scale bar, 500 μm. (E) OCT images of differentiated cells on normal plate (upper panel) and transwell (lower panel). (F) The thicknesses of the differentiated cells on normal plate (right panel) or transwell (left panel) were visualized using a color-scale heatmap from 10 to 90 µm in low-resolution mode. Scale bar, 1 mm. (G) the histogram distribution of the OCT analysis of differentiated cells on normal plate (blue line) and transwell (red line) in low-resolution mode. (H) An in vitro permeability assay using FITC dextran. Fluorescence intensities were quantified by relative fluorescence units (RFU) (n = 3 independent experiments; mean ± SEM, *p < 0.05; two-tailed paired t-test). (I) Immunostaining of UPK II in DE cells (upper panel) and differentiated urothelial cells (lower panel) on a transwell plate (*). Scale bar, 50 μm. (J) Immunostaining of CK20 and p63 in differentiated urothelial cells in the stratified area (upper panel) and less-stratified area (lower panel). Abbreviations: iPSc, induced pluripotent stem cell; DE, definitive endoderm; CHIR, CHIR99021; BPE, bovine pituitary extract; ATRA, all-trans retinoic acid; TZ, troglitazone; PD, PD153035; UPK, uroplakin.