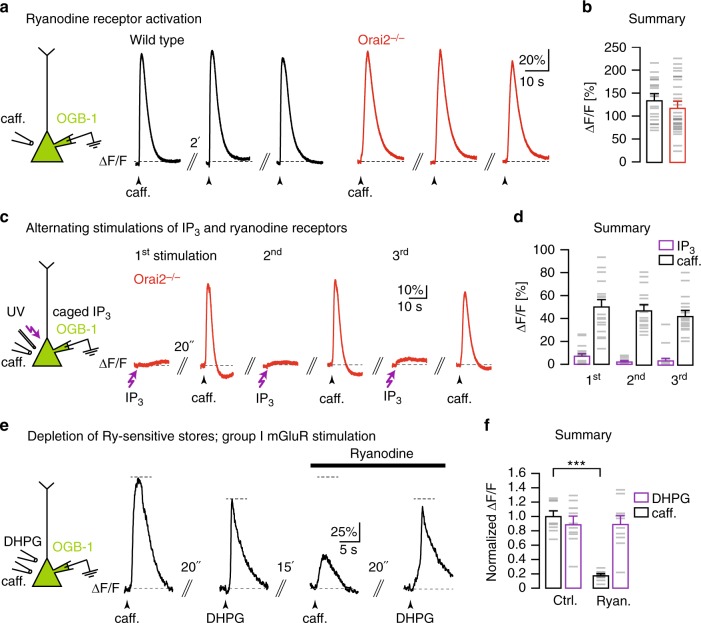
Fig. 2.
Orai2 is not required for RyR-dependent Ca2+ release from internal stores. a Left: Caffeine was locally applied to the somata of whole-cell patch-clamped CA1 pyramidal neurons (PNs) filled with OGB-1 through the patch pipette. Black traces: Fluorescence transients in response to three consecutive caffeine applications with an interval of 2 min in a wild-type mouse. Red traces: Analogous experiment in an Orai2-deficient mouse. b Summary of the experiments in a. Bar graph shows the mean amplitudes of relative fluorescence changes resulting from the first caffeine application in each cell (n = 20 cells in wild-type and n = 28 cells in Orai2−/− mice, p = 0.268). c Left: Both caffeine and ultraviolet pulses were applied to somata of whole-cell patch-clamped CA1 PNs filled through the patch pipette with NPE (caged)-IP3 and OGB-1. Red traces: Fluorescence transients resulting from three cycles of alternating somatic IP3 uncaging and caffeine applications in an Orai2−/− mouse. d Summary of experiments in c. Bar graph showing the mean amplitudes of responses to caffeine (white) and IP3 uncaging (purple; n = 18 cells). e Left: Both caffeine and 3,5-dihydroxyphenylglycine (DHPG) were locally applied to the somata of whole-cell patch-clamped CA1 PNs filled with OGB-1 through the patch pipette. Traces: Fluorescence transients in response to somatic caffeine and DHPG applications with the indicated time intervals in control artificial cerebrospinal fluid (left traces) and in the presence of 10 µM ryanodine (right traces). f Summary bar graph showing mean amplitudes of relative fluorescence changes for all experiments (n = 10 cells, p = 7.89 × 10−9) in e. *** - extremely significant (p < 0.0001)