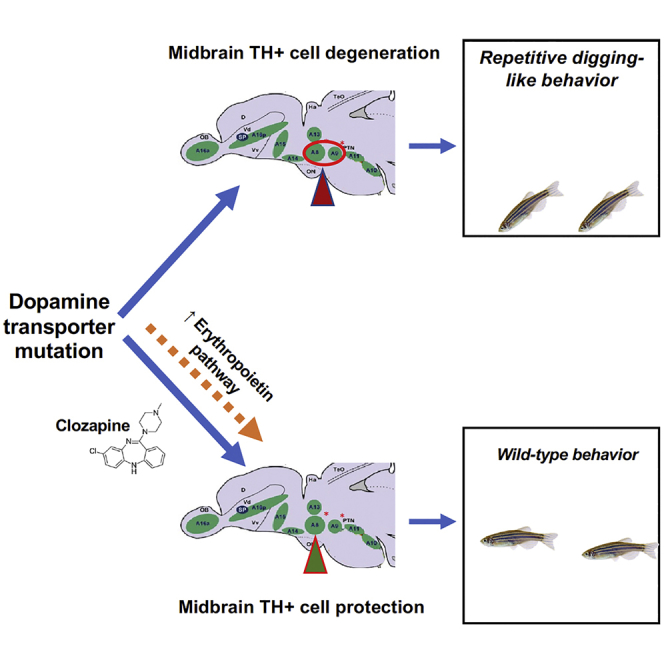
Summary
Dopamine transporter (SLC6A3) deficiency causes infantile Parkinson disease, for which there is no effective therapy. We have explored the effects of genetically deleting SLC6A3 in zebrafish. Unlike the wild-type, slc6a3−/− fish hover near the tank bottom, with a repetitive digging-like behavior. slc6a3−/− fish manifest pruning and cellular loss of particular tyrosine hydroxylase-immunoreactive neurons in the midbrain. Clozapine, an effective therapeutic for treatment-resistant schizophrenia, rescues the abnormal behavior of slc6a3−/− fish. Clozapine also reverses the abnormalities in the A8 region of the mutant midbrain. By RNA sequencing analysis, clozapine increases the expression of erythropoietin pathway genes. Transgenic over-expression of erythropoietin in neurons of slc6a3−/− fish partially rescues the mutant behavior, suggesting a potential mechanistic basis for clozapine's efficacy.
Subject Areas: Biological Sciences, Ethology, Behavioral Neuroscience
Graphical Abstract
Highlights
-
•
DAT mutation in zebrafish causes digging behavior and loss of specific midbrain neurons
-
•
Clozapine restores normal behavior and neuronal morphology of mutant fish
-
•
Clozapine increases expression of erythropoietin pathway genes
-
•
Transgenic expression of erythropoietin partially rescues the mutant behavior
Biological Sciences; Ethology; Behavioral Neuroscience
Introduction
The dopamine transporter (DAT) is responsible for the reuptake of dopamine into presynaptic terminals and is responsible for the termination of dopamine's effect. Because altered dopamine signaling in the brain has been associated with a host of disorders, including Parkinson disease, schizophrenia, bipolar disease, and attention deficit disorder, DAT function has been the focus of genetic studies in several species. In humans, loss of function of DAT causes infantile Parkinson disease, with dystonia, rigidity, bradykinesia, and tremor. Complete loss of function leads to onset in infancy, delayed in some cases until adolescence when there is partial loss of function (Kurian, 1993, Kurian et al., 2011, Ng et al., 2014). DAT deficiency is a rare disorder, but believed to be underdiagnosed (Ng et al., 2014), and there is no effective therapy. DAT coding variants have been associated with attention-deficit/hyperactivity disorder (ADHD) (Mazei-Robison et al., 2005), and polymorphisms in the DAT gene (SLC6A3) have been associated with treatment-resistant schizophrenia (Bilic et al., 2014). Loss-of-function mutation of the DAT gene in mice (Gainetdinov et al., 1998, Giros et al., 1996) and Drosophila (Asjad et al., 2017) causes hyperlocomotion and disordered sleep. In mice, this is associated with reduced levels of releasable dopamine and prolongation of its clearance from the extracellular space (Gainetdinov et al., 1998). DAT-mutant mice have been used as models for ADHD and schizophrenia because of the genetic associations, behaviors, and responses to pharmacological agents (Powell et al., 2008).
We find here that zebrafish with mutation of the gene encoding DAT (slc6a3) hover near the bottom of the tank. We find loss of tyrosine hydroxylase (TH)-immunoreactive neurons, specifically in the A8 midbrain region of the mutant fish. The atypical antipsychotic clozapine, functioning in part through blocking of D2 receptors, reverses the behavioral defect and restores neuronal integrity in the A8 region. RNA sequencing (RNA-seq) analysis indicates that clozapine increases the expression of erythropoietin pathway genes, and transgenic over-expression of Epoa partially rescues the behavior. This suggests that the clozapine function in DAT-mutant fish is at least in part due to the restoration of midbrain TH+ neurons and that it may do so at least in part by upregulating erythropoietin expression.
Results
Fish with Dopamine Transporter Deficiency Display Repetitive Bottom Swimming Behavior
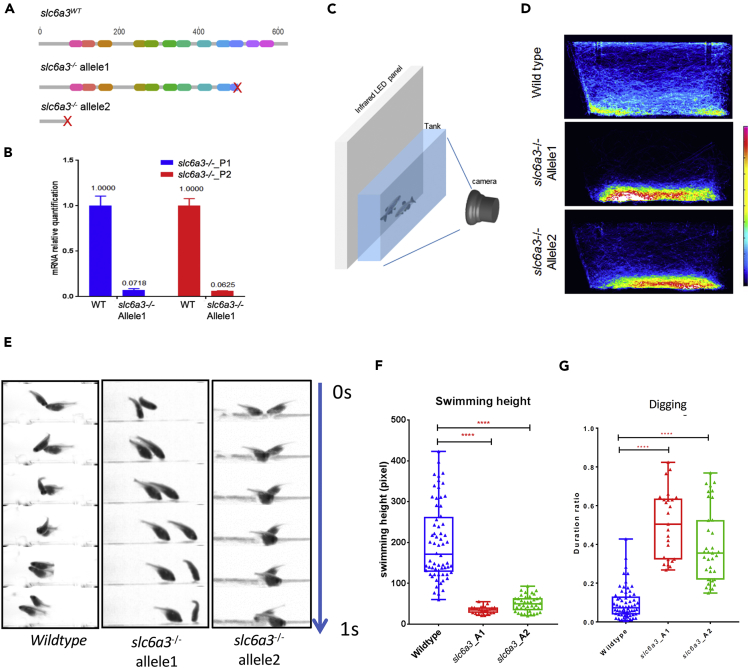
We generated two independent alleles of slc6a3−/− using CRISPR/Cas9 technology (Figures 1A and S1, Table 1). Both cause a predicted frameshift and early termination of protein translation (Figure S1). In addition, allele 1, carrying 7-bp deletion in exon10, has markedly reduced slc6a3 mRNA, presumably from nonsense-mediated mRNA decay (Figure 1B, Table 2). Beginning around 6 weeks post fertilization, slc6a3−/− fish adopt a posture with their bodies at an angle pointing toward the tank bottom (Figures 1C–1E, Videos S1, S2, and S3) and hover toward the bottom of the tank consistently, rarely leaving the lower part of the tank (Figures 1E–1G), although they are fully capable of swimming toward the top of the tank to garner food (data not shown). The fish move back and forth from the bottom of the tank, a behavior reminiscent of the “digging” described previously by Tinbergen and Schemmel in other fish species (Schemmel, 1980, Tinbergen, 1952). The slc6a3−/− fish have normal swimming capability, although the allele2 fish manifest a slight reduction of swimming speed compared with the wild-type (Figure S3A). The tendency for slc6a3−/− fish to dwell toward the bottom of the tank has recently been reported by others (Kacprzak et al., 2017). The slc6a3−/− fish otherwise are indistinguishable from wild-type in terms of morphology, size, and development (Figure S2). Both alleles manifest reduction of spawning rates compared with wild-type: 72% (78 of 109 trials), slc6a3−/− allele 1: 38% (26 of 68 trials), slc6a3−/− allele2: 42% (10 of 24 trials).
Figure 1.
slc6a3 Deficiency Causes Repetitive Behavior
(A) The wild-type Slc6a3 protein structure and two different alleles obtained by CRISPR deletions resulting in truncation at different domains in Slc6a3 proteins.
(B) qPCR indicating dramatic reduction of slc6a3 truncated mRNA in slc6a3−/− allele1 when compared with the wild-type.
(C) The behavior recording system.
(D) Heatmaps of fish trajectory of wild-type and two alleles of slc6a3−/− in the home tank in a 30-min video (60 frames per second). The heatmap colors represent frames that fish appear within each 5 × 5-pixel bin in the image (1224 × 500 pixel in 30 min).
(E) The “digging” behavior in the slc6a3−/− fish compared with wild-type. All 1-s videos show fish at the tank bottom. Fish bearing both slc6a3−/− alleles display a persistent “digging” behavior, whereas wild-type fish do not.
(F) The quantification of average swimming height of fish (nWT = 64, nslc6a3_A1 = 25, nslc6a3_A2 = 40, ****p < 0.0001, significance test: one-way ANOVA Kruskal-Wallis test).
(G) The quantification of duration of “digging” feature over the whole 30-min recording time in the tank using Janelia Automatic Animal Behavior Annotator (JABBA) (Kabra et al., 2013) (nWT = 64, nslc6a3_A1 = 25, nslc6a3_A2 = 40, ****p < 0.0001, significance test: one-way ANOVA Kruskal-Wallis test.)
See Figure S3 for more details.
Table 1.
gRNAs and Genotyping Primers for slc6a3−/− CRISPR Knockout
| Allele1 | Allele2 | |
|---|---|---|
| gRNA | GGAGTACTAATTGAGGCCATCGG | CGTTGAGGTCGGAGCAGTTTGGG |
| Genotyping primer F | GGAGTACTAATTGAGGCCATCGG | CCTTCCCAGACGTCTTCACTCCT |
| Genotyping primer R | ACTTGGGGAAATGTTCATCGTAGG | GATCCTGATCTCGCTGTATGTGG |
Table 2.
Sequences of qRT-PCR Primers
| Forward | Reverse | |
|---|---|---|
| slc6a3-Q1 | GGTTCAGTTCACCTCCTCCA | ACGGACAGCAGAAAGTCGAT |
| slc6a3-Q2 | CCTTCCTCATCTCGCTCATC | TCATCACTGAAGCGATCCAC |
| epoa | TGATGAAGCTTGTCCAGCAC | CAGCTTCCGAGAAAAACCTG |
| epor | GAGGACCAGCGTTCAGACTC | GTGCGAGGCATTTAGAGAGG |
| nt5c2l1 | TGGCGCTCTTACTTTGACCT | CCTGTCGCTTCTTGGACTTC |
| bactin | CGAGCAGGAGATGGGAAC | CAACGGAAACGCTCATTGC |
Midbrain Dopaminergic Neurons Degenerate in slc6a3−/− Fish
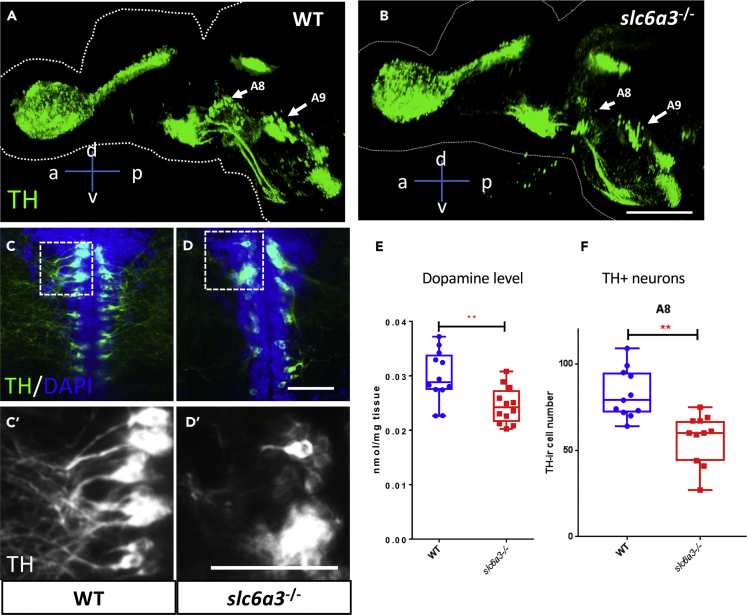
slc6a3 is expressed in the brain in the diencephalon, olfactory bulb, and pretectum (Filippi et al., 2010, and Figure S5A) in larvae and adult. Brain concentrations of dopamine in the slc6a3−/− fish are decreased by 16% compared with wild-type (slc6a3−/− allele1: 0.024 ± 0.001 nmol/mg tissue, wild-type: 0.030 ± 0.001 nmol/mg tissue) (Figure 2E). We therefore wondered if this decline in dopamine might reflect loss or dysfunction of dopaminergic neurons and so labeled cells for TH by immunohistochemistry. As a rate-limiting enzyme of dopamine biosynthesis, TH is often used as a marker for dopaminergic neurons (Wulle and Schnitzer, 1989). We used the CUBIC clearing strategy to visualize TH+ neurons throughout the entire adult brain (Susaki et al., 2015). We find, as have others (Parker et al., 2013), that TH+ neurons are concentrated in a few discrete clusters (Figures 2A and S5B). slc6a3 mutation causes reduction of TH+ neuron number and branching pattern, primarily limited to the midbrain areas termed A8 and A9 in 8-week-old fish brains (Figures 2B–2D, 2C′–D′, 2F, and S5C). TH+ neuron loss in other brain areas was not detected (Figure S5D). The A8 region of the zebrafish midbrain is believed to be homologous to the retrorubral field (RrF) region of mammals, and the A9 region, to the substantia nigra (SNc) (German and Manaye, 1993).
Figure 2.
Midbrain Tyrosine Hydroxylase Immunoreactive (TH+) Neurons Degenerate in the slc6a3−/− Fish Brain
(A and B) Sagittal view of 3-dimensional rendering of tyrosine hydroxylase (TH) immunostaining of the whole brains of (A) wild-type (WT) and (B) slc6a3−/− allelle1 at 3 months post-fertilization. The arrows indicate two groups of TH+ neurons (A8 and A9 areas) in the midbrain and show reduction in cell numbers in the slc6a3−/− allele1 compared with WT. Scale bar, 400 μm. a, anterior; p, posterior; d, dorsal; v, ventral.
(C–E) (C and D) Representative images of TH+ neurons in (C and C′) WT and (D and D′) slc6a3−/− allele1 fish brain in the A8 area at 2 months post-fertilization showing structural changes in neurons and diminution in their arborization. For visualization purpose, images are shown as horizontal ventral views, with anterior on the top. Five optical planes (6-μm interval) were stacked to show the TH+ cells. Scale bar, 20 μm. (E) Mass spectrometry of the dopamine level shows a significant reduction in the slc6a3−/− fish. n = 12 for both WT and slc6a3−/− allele1. **p < 0.01. Significance test: Wilcoxon-Mann-Whitney test.
(F) Boxplot shows reduction in cell numbers of A8 TH+ neurons at 2 months post-fertilization.
Error bar = standard deviation. n = 11 for both WT and slc6a3−/− allele1. **p < 0.01. Significance test: Wilcoxon-Mann-Whitney test.
Chronic Treatment with Clozapine Rescues Wild-Type Behavior and Midbrain TH+ Neurons
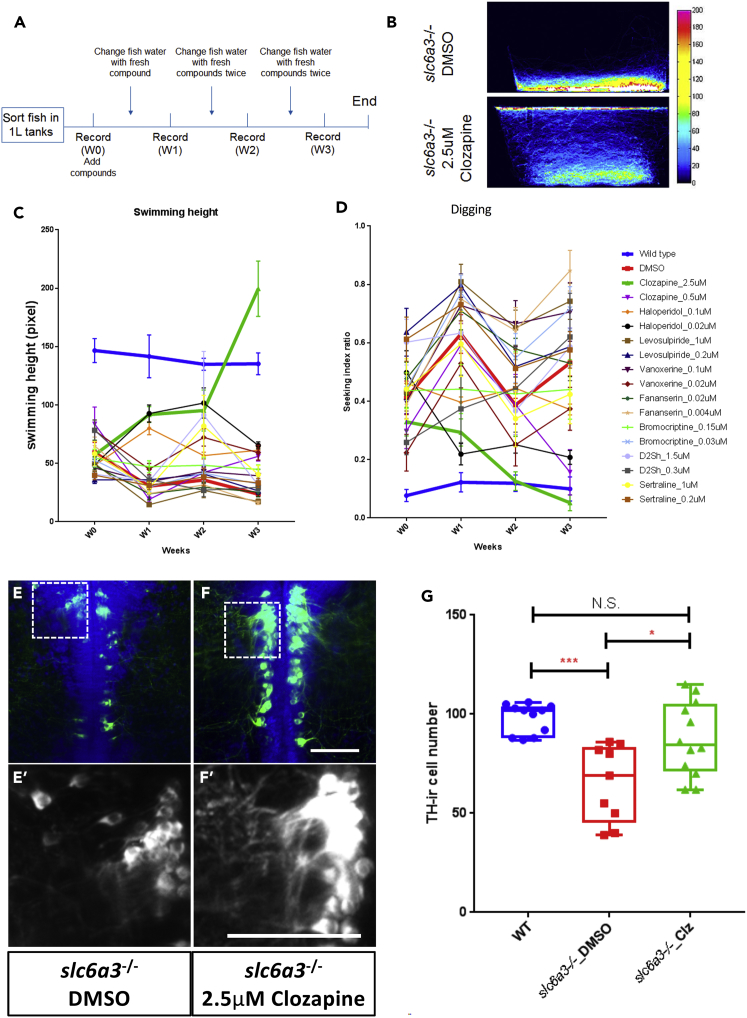
We screened psychoactive compounds, especially those known to act at least in part by affecting dopamine signaling, upon the bottom-dwelling behavior of slc6a3−/− fish. We chose doses predicted to achieve, at final dilution in the water, concentrations of 1× or 5× the EC50 for dopamine receptors and assessed swimming behaviors over time (Figure 3A). Of the compounds, clozapine has the clearest effect on swimming behavior, although it takes at least 3 weeks to manifest (Figures 3B–3D). As shown in Figure 3C, clozapine exposure for 3 weeks causes the fish to resume swimming throughout the height of the tank and, as shown in the attached videos (Videos S4 and S5), completely abrogates the repetitive “digging” behavior (Figures 3D and S4). Thus, clozapine effectively restores the slc6a3−/− behaviors to wild-type.
Figure 3.
Chronic Clozapine Treatment Rescues Aberrant Swimming Behavior and Midbrain TH+ Neurons in the slc6a3−/− Fish Brain
(A) Schematic of the experimental design for chronic treatment of slc6a3−/− allele1 mutant fish with candidate compounds.
(B) The heatmap of fish trajectories of control (DMSO) and 2.5 μM clozapine-treated slc6a3−/− allele1 fish after 3 weeks' treatment showing that clozapine reverses aberrant “digging” behavior.
(C) Time course of swimming height from week 0 (W0) to week 3 (W3) (n = 5; error bar = standard deviation).
(D) The time course of the “digging” feature duration by all compounds over time (n = 5, error bar = standard deviation).
(E–F) Representative images of TH+ neurons in (E and E′) DMSO and (F and F′) 2.5 μM clozapine-treated slc6a3−/− allele1 fish brain in the A8 area at 3 months post-fertilization showing restoration of neuronal number and arborization by clozapine. For visualization purpose, images are shown as horizontal ventral views, with anterior on the top. Five optical planes (6-μm interval) were stacked to show the TH+ cells. Scale bar, 20 μm.
(G) Boxplot shows clozapine-induced restoration of TH-immunoreactive A8 cell number in slc6a3−/− allele1 fish at 3 months post-fertilization.
(For all, n ≥10. Error bar = standard deviation. ***p < 0.001, *p < 0.05. N.S., no significance. Significance test: one-way ANOVA Kruskal-Wallis test).
We also find that 3 weeks of exposure to 2.5 μM clozapine treatment rescues A8 (but not A9) TH+ neuronal number and morphology (3 months post-fertilization, Figures 3E–F′, 3E′–3F′, 3G, and S5E–S5H). This is compatible with the A8 regional loss contributing to the unusual behavior of the mutant fish and suggests that clozapine may act, at least in part, by protecting this population of cells.
Erythropoietin Pathway as a Potential Clozapine Target
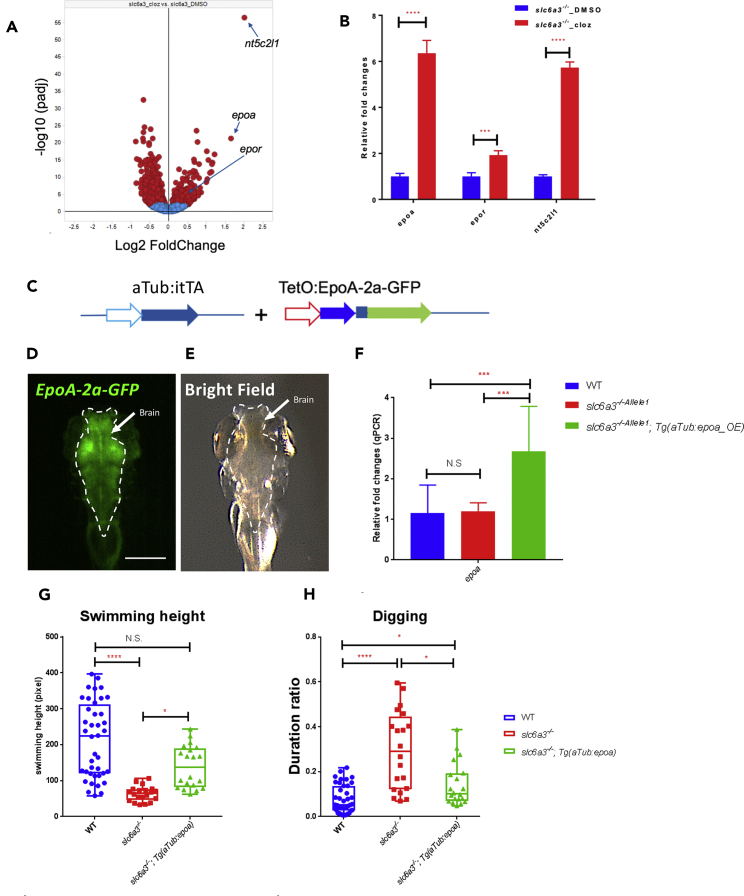
To begin to dissect pathways by which clozapine rescues the slc6a3−/− behavior we performed RNA-seq of the adult slc6a3−/− fish brains and compared clozapine with control treatment (Table S1). Interestingly, levels of dopamine pathway genes are only modestly affected by clozapine treatment (Figure S6A). The most significant changes in expression are in erythropoietin a (epoa, by 6.35 ± 1.62-fold) and 5′-nucleotidase, cytosolic II, like 1 (nt5c2l1 by 5.73 ± 0.69-fold) (Figures 4A and 4B), the latter an enzyme that converts extracellular nucleotides, such as 5′-AMP, to nucleotides, such as adenosine. There are also significant increases in expression of erythropoietin receptor (epor, by 1.92 ± 0.54-fold) (Figures 4A and 4B). Quantitative RT-PCR of these genes independently confirmed the upregulation by clozapine (Figure S6B, Table 2). We focused on the epo pathway because erythropoietin has been previously reported to have neuroprotective effects, particularly on midbrain dopaminergic neurons (Noguchi et al., 2007, Punnonen et al., 2015). We generated a transgenic line stably expressing epoa in CNS neurons (driven by a neuronal alpha tubulin promoter, Figures 4C–4E), and which increases epoa expression levels by more than 2-fold (Figure 4F). When crossed into slc6a3−/− fish, their behavior was restored toward wild-type (Figures 4G and 4H, Video S6), indicating that the clozapine-induced epoa expression may account for part of clozapine's effect in these fish.
Figure 4.
RNA-Seq Analysis Reveals Erythropoietin Pathway as a Potential Clozapine Target
(A) The volcano plot of log2-fold change of gene expression in the adult fish brain between the treatments of clozapine and DMSO in slc6a3−/− allele1. The clozapine highly upregulated genes (epoa, epor, and nt5c2l1) are indicated on the graph.
(B) Bar plot of gene expression of epoa, epor, and nt5c2l1 by RNA-seq analysis. ****p < 0.0001, ***p < 0.001. n = 8 for all. Error bar = standard deviation. Significance test: Wilcoxon-Mann-Whitney test.
(C) The construct maps for over-expression of epoa in neurons with the tetracycline-controlled transcriptional activation system.
(D and E) The dorsal view of the green channel (D) and bright field (E) of a slc6a3−/−_allele1; Tg(aTub:iTTA; TetO:EpoA-2a-GFP) fish at 5 days post-fertilization under a fluorescent dissecting microscope. The brain area (indicated by green fluorescence in D) is outlined by a dotted line. Scale bar, 250 μm.
(F) Whole-brain qRT-PCR at 2 months post-fertilization confirms the upregulation of epoa in the slc6a3−/−_allele1;Tg(aTub:iTTA; TetO:EpoA-2a-GFP) fish. Error bar, standard deviation. n = 6 for all conditions. ***p < 0.001, **p < 0.01, N.S., no significance. Significance test, Wilcoxon-Mann-Whitney test.
(G and H) The quantification of The quantification of average swimming height (G) and duration of “digging” feature (H) of slc6a3−/−_allele1; Tg(aTub:iTTA; TetO:EpoA-2a-GFP) compared to slc6a3−/−_allele1 and wild-type at 2 months post-fertilization.
nWT = 40, nslc6a3−/−_allele1_ = 20, nslc6a3−/−_allele1;tg(aTub:eopa) = 20. ∗∗∗∗p < 0.0001, ∗p < 0.05, N.S., no significance. Significance test: one-way ANOVA Kruskal-Wallis test.
Discussion
We find that homozygous loss of the dopamine uptake transporter, DAT (SLC6A3), causes zebrafish to manifest an unusual repetitive bottom-digging behavior, one reminiscent of sand digging of threatened sticklebacks (Tinbergen, 1952) and bottom searching by cave fish (Astyanax mexicanus) (Schemmel, 1980). Thus, it may be part of a piscine behavioral repertoire, one exaggerated in zebrafish by the mutation. The behavior is accompanied by loss of particular set of midbrain TH+ neurons. Clozapine, an atypical antipsychotic, can restore wild-type behavior and neuronal morphology in one group of the TH neurons. Clozapine increases the expression of erythropoietin pathway genes, and transgenic over-expression of erythropoietin in neurons of slc6a3−/− fish can partially substitute for clozapine in restoring normal behavior.
DAT activity is the primary mechanism for ending dopaminergic transmission, keeping extracellular dopamine levels low, and restoring presynaptic levels. Genetic evidence indicates a role for DAT in both motor and cognitive functions. DAT is the target of the psychostimulants cocaine and amphetamine. In humans, complete loss of function of DAT in humans leads to infantile Parkinson disease, and partial loss, to late-onset dyskinesia (Kurian, 1993, Kurian et al., 2011). DAT polymorphisms or changes in expression levels have been associated with several psychiatric and neurological disorders, including schizophrenia, ADHD, psychostimulant abuse, and Gilles de las Tourette syndrome (Kurian, 1993, Kurian et al., 2011).
We noted no gross morphological change in the slc6a3−/− brain. This is consistent with the MRI scan in patients with DAT deficiency syndrome (Ng et al., 2014). The most prominent effect we noted in the slc6a3−/− fish is neuronal loss and dysmorphology of TH-expressing neurons primarily in the A8 and A9 regions of the midbrain. These are believed to be homologous to RrF and SNc regions of the mammalian brain, respectively (Arenas et al., 2015, Parker et al., 2013). TH+ neurons of the SNc project to the caudate putamen and dorsolateral striatum, and their loss in Parkinson disease is believed to be responsible for the gait abnormalities in this disorder (Arenas et al., 2015). The TH+ neurons of the A8 homologous region, the RrF, project to the nucleus accumbens and the limbic cerebral cortex and have been proposed to be involved in emotion, reward, and cognitive function, and to be affected in a range of psychiatric disorders (Arenas et al., 2015, Gallo et al., 2018). Abnormalities of both the nucleus accumbens and limbic cerebral cortex are implicated in schizophrenia pathology by postmortem human tissue analysis (Jakob and Beckmann, 1986, McCollum and Roberts, 2015). Interestingly, slc6a3-mutant mice manifest only minor reductions in TH-immunoreactive neurons in the striatum, the region most affected (Cyr et al., 2003). This may reflect a species difference, or it may be that, as in the fish, it is only a small subpopulation of specific neurons that are lost in the mouse.
Clozapine was synthesized originally with the goal of avoiding the extrapyramidal side effects of earlier antipsychotic drugs, assumed accomplished by diminishing its D2 activity. However, it is clear that its therapeutic mechanism is unlikely to be explained solely by its effect upon the dopamine system, because it also enhances the activity at serotonin 2A receptor and several other receptors (Crilly, 2007). Clozapine's use is compromised by rare but life-threatening agranulocytosis, and more common weight gain, so understanding its mechanism of action is critical to the development of efficacious and safer therapeutics. The zebrafish brain can regenerate neurons in response to injury (Fleisch et al., 2011, Ghosh and Hui, 2016), and it is certainly conceivable that human responses to clozapine might not include the dramatic repair that we see here.
We find that epoa and epo receptors are among the genes whose expressions are most increased by clozapine and that transgenic epoa expression in neurons can mirror clozapine's restorative effect on behavior. These observations are of interest because, in addition to its well-defined role in erythropoiesis, the Epo pathway has been proposed as neuroprotective in a variety of CNS disorders, including ischemia, traumatic brain injury, Parkinson disease, and schizophrenia (Kastner et al., 2012, Punnonen et al., 2015). In the CNS, EpoRs are expressed by neurons, glia, and endothelial cells, especially during embryonic development (Noguchi et al., 2007). Mice lacking Epo or its receptor have defective neurogenesis during development (Noguchi et al., 2007). Adult TH+ neurons express EpoR, and Epo can rescue neurons from 6-hydroxydopamine toxicity, both in vitro and in vivo by CNS infusion (Punnonen et al., 2015). It is believed that Epo actions in the CNS are mediated by a heterodimer of the EpoR and CD131, rather than via the homodimer responsible for Epo activation of erythropoiesis (Punnonen et al., 2015). Mutation of slc6a3 does not by itself affect epoa levels, so we presume that Epo is not in the pathway directly affected by slc6a3 mutation and that salvage by transgenic over-expression of Epo is likely to act by a pathway parallel to that perturbed in slc6a3−/− fish.
We speculate that damage to TH+ neurons in the A8 (and A9) region of the zebrafish midbrain is responsible for the bottom-digging-like repetitive behavior of the slc6a3−/− fish, and that clozapine rescue of the A8 neural cells is responsible for restoring their behavior toward that of wild-type. Interestingly, we do not see significant rescue of A9 by clozapine treatment. Of course, we cannot determine whether the rescue of TH+ neurons also accounts for clozapine's therapeutic effect in humans, although the areas perturbed in schizophrenia include those believed homologous to projection zones of A8-equivalent (i.e., RrF) DA neurons. In addition, the similar restorative effect exerted by erythropoietin expression suggests that this might be a novel pathway to explore for discovery of new medicines with clozapine-like therapeutic activities.
Limitations of the Study
The observations suggest that the behavioral disorder may be due to A8 neuronal dysfunction, but proof will require specific targeting of such neurons, unfortunately technically non-trivial because the midbrain is relatively deep in animals old enough to manifest this behavioral phenotype.
We have focused upon the Epo pathway as one potential intermediary in the clozapine effect. Transgenic epoa expression rescues behavior in slc6a3−/− animals, but not to the same degree as clozapine, suggesting that we may need to target more precisely or that it explains only part of clozapine's effect. Along these lines it would be of interest to mutate epoa to see if such mutation generates behavior akin to that seen in slc6a3−/− fish, but the critical role of Epo in hematopoiesis causes early death in such animals. Genes other than epoa are regulated by clozapine and could be important. For example, there is an increase in 5′-nucleotidase (an enzyme that converts extracellular nucleotides, such as 5′-AMP, to nucleotide, such as adenosine), the activity of which is known to increase after clozapine treatment in humans and in the rat (Brunstein et al., 2007, Lara et al., 2001).
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Ajeet Singh, Alexis Hubaud, and Jian Fang for helpful discussions and scientific input on the manuscripts. We thank Stephanie Wiessner, Gregory Molind, Ellen Sanchez, Carmen Diaz Verdugo, Jingyao Li, and Wenlong Tang for helpful discussions and help with reagents and hardware. We thank Ned Kirkpatrick and Novartis Imaging Core for helping with microscopy, Carsten Russ and Novartis NGS facility for sequencing, and Kara Maloney and Novartis zebrafish facility for animal care.
Author Contributions
G.W., P.Z., and M.C.F. conceived the project; G.W., G.Z., D.J.G., and M.C.F. designed experiments; G.W., Z.L., and C.H.F. performed most of the experiments; G.Z., Z.L., and M.C. generated the mutant alleles; G.W. and C.H.F. performed immunofluorescence imaging and data analysis; G.W., G.Z., Z.L., and M.X.S. performed the RNA-seq experiment and data analysis; G.W. and Z.L. generated over-expression constructs and performed injection; G.W. and T.T. performed behavior data acquisition and analysis; K.M. and S.J.T. performed mass spectrometry experiment and analysis; P.Z., D.J.G., and M.C.F. supervised the project; G.W., D.J.G., and M.C.F. wrote the paper with input from all authors.
Declaration of Interests
M.C.F. is an advisor to Novartis, MPM, and Burrage Capital and member of the Board of Directors of Semma Therapeutics and Beam Therapeutics, and of the SAB of Tenaya Therapeutics. All other authors were Novartis employees at the time study was conducted. D.J.G. is an employee of and stockholder in Novartis. This study was funded by Novartis AG.
Published: July 26, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.06.039.
Supplemental Information
References
- Arenas E., Denham M., Villaescusa J.C. How to make a midbrain dopaminergic neuron. Development. 2015;142:1918–1936. doi: 10.1242/dev.097394. [DOI] [PubMed] [Google Scholar]
- Asjad H.M.M., Kasture A., El-Kasaby A., Sackel M., Hummel T., Freissmuth M., Sucic S. Pharmacochaperoning in a Drosophila model system rescues human dopamine transporter variants associated with infantile/juvenile parkinsonism. J. Biol. Chem. 2017;292:19250–19265. doi: 10.1074/jbc.M117.797092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilic P., Jukic V., Vilibic M., Savic A., Bozina N. Treatment-resistant schizophrenia and DAT and SERT polymorphisms. Gene. 2014;543:125–132. doi: 10.1016/j.gene.2014.03.050. [DOI] [PubMed] [Google Scholar]
- Brunstein M.G., Silveira E.M., Jr., Chaves L.S., Machado H., Schenkel O., Belmonte-de-Abreu P., Souza D.O., Lara D.R. Increased serum adenosine deaminase activity in schizophrenic receiving antipsychotic treatment. Neurosci. Lett. 2007;414:61–64. doi: 10.1016/j.neulet.2006.11.071. [DOI] [PubMed] [Google Scholar]
- Crilly J. The history of clozapine and its emergence in the US market: a review and analysis. Hist. Psychiatry. 2007;18:39–60. doi: 10.1177/0957154X07070335. [DOI] [PubMed] [Google Scholar]
- Cyr M., Beaulieu J.M., Laakso A., Sotnikova T.D., Yao W.D., Bohn L.M., Gainetdinov R.R., Caron M.G. Sustained elevation of extracellular dopamine causes motor dysfunction and selective degeneration of striatal GABAergic neurons. Proc. Natl. Acad. Sci. U S A. 2003;100:11035–11040. doi: 10.1073/pnas.1831768100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi A., Mahler J., Schweitzer J., Driever W. Expression of the paralogous tyrosine hydroxylase encoding genes th1 and th2 reveals the full complement of dopaminergic and noradrenergic neurons in zebrafish larval and juvenile brain. J. Comp. Neurol. 2010;518:423–438. doi: 10.1002/cne.22213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisch V.C., Fraser B., Allison W.T. Investigating regeneration and functional integration of CNS neurons: lessons from zebrafish genetics and other fish species. Biochim. Biophys. Acta. 2011;1812:364–380. doi: 10.1016/j.bbadis.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Gainetdinov R.R., Jones S.R., Fumagalli F., Wightman R.M., Caron M.G. Re-evaluation of the role of the dopamine transporter in dopamine system homeostasis. Brain Res. Brain Res. Rev. 1998;26:148–153. doi: 10.1016/s0165-0173(97)00063-5. [DOI] [PubMed] [Google Scholar]
- Gallo E.F., Meszaros J., Sherman J.D., Chohan M.O., Teboul E., Choi C.S., Moore H., Javitch J.A., Kellendonk C. Accumbens dopamine D2 receptors increase motivation by decreasing inhibitory transmission to the ventral pallidum. Nat. Commun. 2018;9:1086. doi: 10.1038/s41467-018-03272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German D.C., Manaye K.F. Midbrain dopaminergic neurons (nuclei A8, A9, and A10): three-dimensional reconstruction in the rat. J. Comp. Neurol. 1993;331:297–309. doi: 10.1002/cne.903310302. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Hui S.P. Regeneration of zebrafish CNS: adult neurogenesis. Neural Plast. 2016;2016:5815439. doi: 10.1155/2016/5815439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giros B., Jaber M., Jones S.R., Wightman R.M., Caron M.G. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Jakob H., Beckmann H. Prenatal developmental disturbances in the limbic allocortex in schizophrenics. J. Neural Transm. 1986;65:303–326. doi: 10.1007/BF01249090. [DOI] [PubMed] [Google Scholar]
- Kabra M., Robie A.A., Rivera-Alba M., Branson S., Branson K. JAABA: interactive machine learning for automatic annotation of animal behavior. Nat. Methods. 2013;10:64–67. doi: 10.1038/nmeth.2281. [DOI] [PubMed] [Google Scholar]
- Kacprzak V., Patel N.A., Riley E., Yu L., Yeh J.J., Zhdanova I.V. Dopaminergic control of anxiety in young and aged zebrafish. Pharmacol. Biochem. Behav. 2017;157:1–8. doi: 10.1016/j.pbb.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner A., Grube S., El-Kordi A., Stepniak B., Friedrichs H., Sargin D., Schwitulla J., Begemann M., Giegling I., Miskowiak K.W. Common variants of the genes encoding erythropoietin and its receptor modulate cognitive performance in schizophrenia. Mol. Med. 2012;18:1029–1040. doi: 10.2119/molmed.2012.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian M.A. SLC6A3-related dopamine transporter deficiency syndrome. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J.H., Stephens K., Amemiya A., editors. GeneReviews((R)) University of Washington; Seattle: 1993. https://www.ncbi.nlm.nih.gov/books/NBK442323/ [Google Scholar]
- Kurian M.A., Li Y., Zhen J., Meyer E., Hai N., Christen H.J., Hoffmann G.F., Jardine P., von Moers A., Mordekar S.R. Clinical and molecular characterisation of hereditary dopamine transporter deficiency syndrome: an observational cohort and experimental study. Lancet Neurol. 2011;10:54–62. doi: 10.1016/S1474-4422(10)70269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara D.R., Vianna M.R., de Paris F., Quevedo J., Oses J.P., Battastini A.M., Sarkis J.J., Souza D.O. Chronic treatment with clozapine, but not haloperidol, increases striatal ecto-5'-nucleotidase activity in rats. Neuropsychobiology. 2001;44:99–102. doi: 10.1159/000054925. [DOI] [PubMed] [Google Scholar]
- Mazei-Robison M.S., Couch R.S., Shelton R.C., Stein M.A., Blakely R.D. Sequence variation in the human dopamine transporter gene in children with attention deficit hyperactivity disorder. Neuropharmacology. 2005;49:724–736. doi: 10.1016/j.neuropharm.2005.08.003. [DOI] [PubMed] [Google Scholar]
- McCollum L.A., Roberts R.C. Uncovering the role of the nucleus accumbens in schizophrenia: a postmortem analysis of tyrosine hydroxylase and vesicular glutamate transporters. Schizophr. Res. 2015;169:369–373. doi: 10.1016/j.schres.2015.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng J., Zhen J., Meyer E., Erreger K., Li Y., Kakar N., Ahmad J., Thiele H., Kubisch C., Rider N.L. Dopamine transporter deficiency syndrome: phenotypic spectrum from infancy to adulthood. Brain. 2014;137:1107–1119. doi: 10.1093/brain/awu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi C.T., Asavaritikrai P., Teng R., Jia Y. Role of erythropoietin in the brain. Crit. Rev. Oncol. Hematol. 2007;64:159–171. doi: 10.1016/j.critrevonc.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M.O., Brock A.J., Walton R.T., Brennan C.H. The role of zebrafish (Danio rerio) in dissecting the genetics and neural circuits of executive function. Front. Neural Circuits. 2013;7:63. doi: 10.3389/fncir.2013.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell S.B., Young J.W., Ong J.C., Caron M.G., Geyer M.A. Atypical antipsychotics clozapine and quetiapine attenuate prepulse inhibition deficits in dopamine transporter knockout mice. Behav. Pharmacol. 2008;19:562–565. doi: 10.1097/FBP.0b013e32830dc110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punnonen J., Miller J.L., Collier T.J., Spencer J.R. Agonists of the tissue-protective erythropoietin receptor in the treatment of Parkinson's disease. Curr. Top. Med. Chem. 2015;15:955–969. doi: 10.2174/156802661510150328224527. [DOI] [PubMed] [Google Scholar]
- Schemmel C. Studies on the genetics of feeding behaviour in the cave fish Astyanax mexicanus f. anoptichthys. An example of apparent monofactorial inheritance by polygenes. Z. Tierpsychol. 1980;53:9–22. doi: 10.1111/j.1439-0310.1980.tb00730.x. [DOI] [PubMed] [Google Scholar]
- Susaki E.A., Tainaka K., Perrin D., Yukinaga H., Kuno A., Ueda H.R. Advanced CUBIC protocols for whole-brain and whole-body clearing and imaging. Nat. Protoc. 2015;10:1709–1727. doi: 10.1038/nprot.2015.085. [DOI] [PubMed] [Google Scholar]
- Tinbergen N. Derived activities; their causation, biological significance, origin, and emancipation during evolution. Q. Rev. Biol. 1952;27:1–32. doi: 10.1086/398642. [DOI] [PubMed] [Google Scholar]
- Wulle I., Schnitzer J. Distribution and morphology of tyrosine hydroxylase-immunoreactive neurons in the developing mouse retina. Brain Res. Dev. Brain Res. 1989;48:59–72. doi: 10.1016/0165-3806(89)90093-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.