Abstract
Aim
This study aimed to investigate the correlation of lnc‐ANRIL/miR‐125a axis with risk, severity, inflammation, and prognosis of sepsis.
Methods
A hundred and twenty‐six sepsis patients and 125 healthy controls were recruited, and then, blood samples were collected, and plasma was separated for lnc‐ANRIL, miR‐125a, lnc‐ANRIL/miR‐125a axis, and inflammatory cytokine level detections. In addition, basic characteristics, 28‐day mortality, and accumulating survival of sepsis patients were recorded.
Results
Plasma lnc‐ANRIL expression was increased, miR‐125a expression was decreased, and lnc‐ANRIL/miR‐125a axis level was elevated in sepsis patients compared with healthy controls, and all of them had good value for predicting sepsis risk with AUCs of 0.800, 0.817, and 0.843, respectively. Lnc‐ANRIL and lnc‐ANRIL/miR‐125a axis were positively correlated with biochemical index levels including CRP and PCT levels, disease severity scale scores, and pro‐inflammatory cytokine levels in sepsis patients, while miR‐125a displayed the opposite trend. Lnc‐ANRIL and lnc‐ANRIL/miR‐125a axis expressions were elevated, while miR‐125a expression was declined in deaths compared with survivors, and all of them predicted 28‐day mortality in sepsis patients with AUCs of 0.765, 0.745, and 0.785, respectively. Subsequently, the Kaplan‐Meier analysis revealed that patients with high lnc‐ANRIL, low miR‐125a, and high lnc‐ANRIL/miR‐125a axis levels presented with worse accumulating survival. In addition, multivariate regression model analyses revealed that high plasma lnc‐ANRIL/miR‐125a axis was an independent predictive factor for both increased 28‐day mortality and worse accumulating survival.
Conclusion
Circulating lnc‐ANRIL/miR‐125a axis was upregulated and could serve as a biomarker for severity, inflammation, and prognosis in sepsis patients.
Keywords: inflammation, Lnc‐ANRIL/miR‐125a axis, prognosis, risk, sepsis, severity
1. INTRODUCTION
Sepsis, a common cause of global deaths, attacks roughly 2.8 million patients every year in developed countries, and the incidence is predicted to increase in the future due to the aging population worldwide.1 A critical issue of sepsis is that patients are at extremely high risk of developing multiple organ dysfunction syndromes (MODS), which is a typical outcome of systemic inflammatory response syndrome (SIRS) and normally leads to deaths ultimately.2 Although a new definition of sepsis had been made by the Third International Consensus Definition for Sepsis and Septic Shock (Sepsis‐3) to describe better the disease and mounting risk factors have been discovered to identify the disease, the diagnosis of sepsis is still not satisfactory due to that the signs and current biomarkers of sepsis are not specific and sensitive enough.3, 4, 5, 6 Therefore, patients who develop sepsis are still facing high risk of mortality and disability even if the patients recover, which is due to that the intervention is often late.
Recently, the wide application of high‐throughput sequencing benefiting from the progress in technology and decrease in cost has led to incremental improvements in the exploration of sepsis pathogenesis, among which noncoding RNA takes a critical place in the participation of inflammation, immune disorder, and organ dysfunction in sepsis.7, 8, 9, 10 With respect to the values of noncoding RNAs to be biomarkers or therapeutic targets in sepsis patients, long noncoding RNA (lncRNA) and microRNA (miRNA) are the most implicated ones.11, 12 Lnc‐antisense noncoding RNA in the INK4 locus (ANRIL) is a lncRNA located on chromosome 9p21 region and transcribed by RNA polymerase II and is indicated in the regulation of inflammation and immune responses.13, 14 miR‐125a, a highly reserved miRNA locating on chromosome 19, 11, and 21, is also closely related to inflammation and immunity, and this miRNA has been reported to have the potential in regulating sepsis pathogenesis.15, 16 Moreover, it is a direct target of lnc‐ANRIL, and the lnc‐ANRIL/miR‐125a axis has been indicated in many diseases.17, 18, 19, 20, 21 However, the role of lnc‐ANRIL/miR‐125a axis in sepsis remains unknown.
Thus, this study aimed to investigate the correlation of lnc‐ANRIL/miR‐125a axis with risk, severity, inflammation, and prognosis of sepsis.
2. METHODS
2.1. Sepsis patients and healthy controls
In the current study, 126 sepsis patients and 125 healthy controls were recruited from The Central Hospital of Wuhan between January 2015 and April 2018. For the sepsis patients, inclusion criteria were as follows (a) diagnosed as sepsis according to the criteria of 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference22 and (b) age ≥18 years. The exclusion criteria were (a) died within 24 hours after admitted to our hospital; (b) concomitant with malignancies; (c) infected with human immunodeficiency virus (HIV); (d) history of radiotherapy and chemotherapy within 6 months; (e) treated with immunosuppressant within 1 month; (f) pregnant or lactating women. As for the healthy controls, their health conditions were confirmed by the medical examinations before enrollment, and all of them were older than 18 years. Approval of the present study was obtained from the Ethics Committee of The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology. All participants or their guardians provided written informed consents.
2.2. Blood sample collection
Collection of enrolled sepsis patients' blood sample was performed within 24 hours after hospital admission, and the blood samples of healthy controls were extracted after the acquisition of the written informed consents. All collected blood samples were centrifugated at 3500 r/min, 10 min, 4°C to separate plasma within 1 hour after collection, and then, isolated plasma was stored at −80°C until further determination.
2.3. Detection of lnc‐ANRIL and miR‐125a
First of all, total RNA was extracted from plasma by the QIAamp RNA Blood Mini Kit (Qiagen). After the concentration, purity and integrity of the total RNA were evaluated, and the total RNA was reversely transcribed into complementary DNA using PrimeScript™ RT reagent Kit (Takara). Second, quantitative polymerase chain reaction (qPCR) was performed by using KOD SYBR® qPCR Mix (Toyobo). GAPDH was used as an internal reference for lnc‐ANRIL detection, and U6 was used as an internal reference for miR‐125a detection. Finally, the results were calculated by the formula. Primers used in the qPCR were as follows: lnc‐ANRIL: forward: TGCTCTATCCGCCAATCAGG, reverse: GGGCCTCAGTGGCACATACC; miR‐125a: forward: ACACTCCAGCTGGGTCCCTGAGACCCTTTAAC, reverse: TGTCGTGGAGTCGGCAATTC; GAPDH: forward: TGACCACAGTCCATGCCATCAC, reverse: GCCTGCTTCACCACCTTCTTGA; U6: forward: CTCGCTTCGGCAGCACATATACTA, reverse: ACGAATTTGCGTGTCATCCTTGC.
2.4. Detection of inflammatory cytokines
The levels of inflammatory cytokines in plasma of sepsis patients were detected by enzyme‐linked immunosorbent assay (ELISA) using human ELISA Kits (R&D Systems Inc), and the inflammatory cytokines included tumor necrosis factor (TNF‐α), interleukin‐6 (IL‐6), IL‐8, and IL‐17. All the sample processing and detection procedures of ELISA were carried out in strict accordance with the manufacturer's instructions.
2.5. Data collection
Basic characteristics of sepsis patients were recorded in detail postenrollment, which consisted of (a) demographic information: age, gender, and body mass index (BMI); (b) biochemical indexes: serum creatinine (Scr), albumin, white blood cell (WBC), C‐reactive protein (CRP), and procalcitonin (PCT); (c) disease severity: acute physiology and chronic health evaluation (APACHE) II score and sequential organ failure assessment (SOFA) score, which were assessed within 24 hours after hospital admission.
2.6. 28‐day mortality assessment
Conventional treatments were administered to sepsis patients after hospital admission, with 28‐day follow‐up duration. And the 28‐day mortality was calculated as well. Accumulating survival was defined as the time from the day of hospital admission to the day of death in the hospital or last visit.
2.7. Statistical analysis
SPSS 20.0 (SPSS Inc) was used for statistical analysis, and GraphPad Prism 7.02 (GraphPad Software) was applied for graphs plotting. Continuous data were displayed as mean ± standard deviation (SD) or median (interquartile range (IQR)); count data were expressed as count (percentage). Differences between two groups were determined by Wilcoxon rank‐sum test. Correlations between variables were analyzed using the Spearman's rank test. Variables' diagnostic value for sepsis and predicting value for death were assessed by receiver operating characteristic (ROC) curve and the derived area under the curve (AUC). Variables affecting 28‐day mortality were screened using univariate and multivariate logistic regression model analyses. Accumulating survival was illustrated using Kaplan‐Meier curve, and the difference of which was determined by the log‐rank test. Variables affecting accumulating survival were evaluated by the univariate and multivariate Cox's proportional hazards model analyses. P value < 0.05 was considered as significant.
3. RESULTS
3.1. Patients' characteristics
The sepsis patients in our study included 83 males and 43 females, who had a mean age of 56.6 ± 13.0 years and a mean BMI of 23.1 ± 5.5 kg/m2 (Table 1). The mean values of APACHE II score and SOFA score in sepsis patients were 12.9 ± 5.5 and 5.1 ± 2.8, respectively. In addition, the median values of TNF‐α, IL‐6, IL‐8, IL‐17, lnc‐ANRIL, miR‐125a, and lnc‐ANRIL/miR‐125a axis levels in plasma were 195.0 (119.3‐285.1) pg/mL, 61.9 (36.5‐132.4) pg/mL, 54.6 (25.8‐113.1) pg/mL, 129.4 (71.1‐188.9) pg/mL, 2.56 (1.02‐4.98), 0.30 (0.13‐0.59), and 9.37 (1.87‐24.33), respectively. Other information of biochemical indexes is presented in Table 1.
Table 1.
Characteristics of sepsis patients
| Characteristics | Sepsis patients (N = 126) |
|---|---|
| Demographic information | |
| Age (y), mean ± SD | 56.6 ± 13.0 |
| Gender (male/female) | 83/43 |
| BMI (kg/m2), mean ± SD | 23.1 ± 5.5 |
| Biochemical index, median (IQR) | |
| Scr (mg/dL) | 1.7 (1.2‐2.4) |
| Albumin (g/L) | 24.7 (20.57‐32.17) |
| WBC (109/L) | 18.4 (3.5‐28.1) |
| CRP (mg/L) | 103.6 (52.3‐141.0) |
| PCT (ng/mL) | 14.2 (7.8‐24.7) |
| Disease severity, mean ± SD | |
| APACHE II score | 12.9 ± 5.5 |
| SOFA score | 5.1 ± 2.8 |
| Inflammatory cytokine, median (IQR) | |
| TNF‐α (pg/mL) | 195.0 (119.3‐285.1) |
| IL‐6 (pg/mL) | 61.9 (36.5‐132.4) |
| IL‐8 (pg/mL) | 54.6 (25.8‐113.1) |
| IL‐17 (pg/mL) | 129.4 (71.1‐188.9) |
| lnc‐ANRIL relative expression, median (IQR) | 2.56 (1.02‐4.98) |
| miR‐125a relative expression, median (IQR) | 0.30 (0.13‐0.59) |
| lnc‐ANRIL/miR‐125a axis, median (IQR) | 9.37 (1.87‐24.33) |
Abbreviations: APACHE II, acute physiology and chronic health evaluation II; BMI, body mass index; CRP, C‐reactive protein; IL, interleukin; IQR, interquartile range; PCT, procalcitonin; Scr, serum creatinine; SD, standard deviation; SOFA, sequential organ failure assessment; TNF‐α, tumor necrosis factor‐α; WBC, white blood cell.
3.2. Values of plasma lnc‐ANRIL/miR‐125a axis in predicting sepsis risk
Plasma lnc‐ANRIL expression was elevated (P < 0.001; Figure 1A), while miR‐125a expression was declined (P < 0.001; Figure 1B) in sepsis patients compared with healthy controls. The lnc‐ANRIL/miR‐125a axis was increased in sepsis patients than that in healthy controls (P < 0.001; Figure 1C). ROC curve analyses revealed that lnc‐ANRIL and miR‐125a expressions had good predictive values for sepsis risk with AUCs of 0.800 (95% CI: 0.745‐0.854) and 0.817 (95% CI: 0.764‐0.870), and lnc‐ANRIL/miR‐125a axis exhibited numerically increased value for sepsis risk with AUC 0.843 (95CI: 0.793‐0.892; Figure 1D). In addition, the lnc‐ANRIL expression was negatively correlated with miR‐125a expression in sepsis patients (P < 0.001, r = −0.375; Figure 2A), and lnc‐ANRIL expression was also negatively associated with miR‐125a level in healthy controls but with a lower correlation coefficient value (P = 0.024, r = −0.203; Figure 2B).
Figure 1.
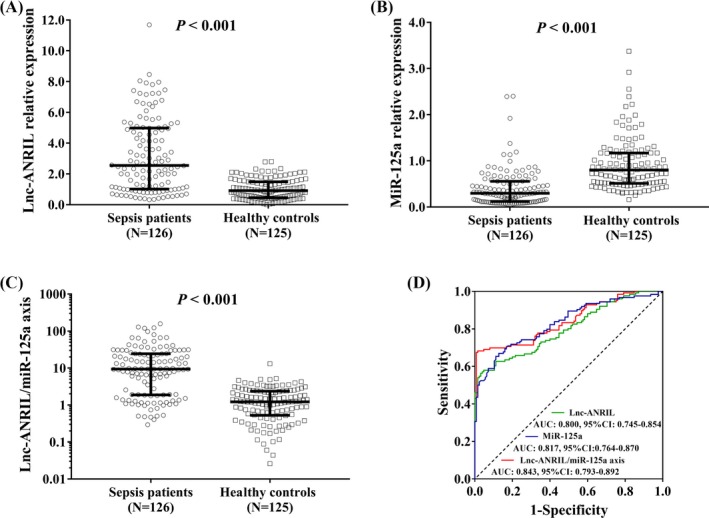
The predictive value of lnc‐ANRIL/miR‐125a axis for sepsis risk. Plasma lnc‐ANRIL expression was increased (A), miR‐125a expression was decreased (B), and lnc‐ANRIL/miR‐125a axis was upregulated (C) in sepsis patients compared with those in healthy controls. ROC curve analyses revealed that lnc‐ANRIL, miR‐125a, and lnc‐ANRIL/miR‐125a axis had good predictive values for sepsis risk (D). Comparison between two groups was determined by Wilcoxon rank‐sum test. Variables' diagnostic value for sepsis and predicting value for death were assessed by ROC curve and the derived area AUC P value < 0.05 was considered as significant. ANRIL, antisense noncoding RNA in the INK4 locus; ROC, receiver operating characteristic; AUC, area under the curve
Figure 2.
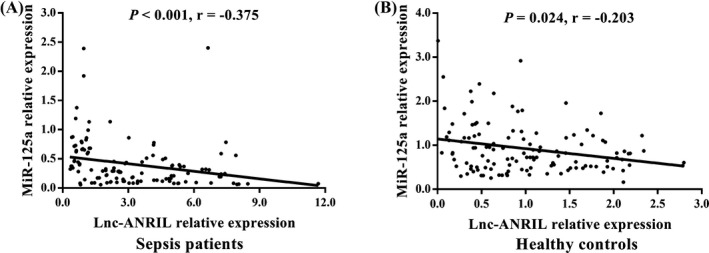
Correlation of lnc‐ANRIL expression with miR‐125a expression. Plasma lnc‐ANRIL expression was reversely correlated with miR‐125a expression in sepsis patients (A) and in healthy controls (B). Correlations between variables were analyzed using the Spearman's rank test. P value < 0.05 was considered as significant. ANRIL, antisense noncoding RNA in the INK4 locus
3.3. Correlation of lnc‐ANRIL/miR‐125a axis with severity and inflammation in sepsis patients
Lnc‐ANRIL expression in plasma was positively correlated with CRP (P = 0.002, r = 0.273), PCT (P = 0.008, r = 0.235), APACHE II score (P < 0.001, r = 0.291), SOFA score (P < 0.001, r = 0.375), TNF‐α expression (P = 0.001, r = 0.283), and IL‐8 expression (P = 0.001, r = 0.294) in sepsis patients (Table 2). MiR‐125a expression was negatively associated with Scr (P = <0.001, r = −0.306), WBC (P = 0.048, r = −0.176), CRP (P = 0.001, r = −0.289), APACHE II score (P = 0.001, r = −0.292), SOFA score (P = 0.009, r = 0.233), TNF‐α (P = 0.002, r = −0.273), IL‐6 (P = 0.002, r = −0.276), IL‐8 (P = 0.003, r = −0.262), and IL‐17 (P < 0.001, r = −0.312) levels. Most importantly, the lnc‐ANRIL/miR‐125a axis level was positively correlated with Scr (P = <0.001, r = 0.310), WBC (P = 0.024, r = 0.201), CRP (P < 0.001, r = 0.335), PCT (P = 0.009, r = 0.231), APACHE II score (P < 0.001, r = 0.354), SOFA score (P < 0.001, r = 0.365), TNF‐α (P < 0.001, r = 0.318), IL‐6 (P = 0.010, r = 0.229), IL‐8 (P < 0.001, r = 0.323), and IL‐17 (P < 0.004, r = 0.252) expressions. However, several of the correlations had relatively low correlation coefficient (below 0.3), which might indicate less strong associations.
Table 2.
Correlation of RNAs relative expression with clinical characteristics
| Clinical characteristics | lnc‐ANRIL | miR‐125a | lnc‐ANRIL/miR‐125a axis | |||
|---|---|---|---|---|---|---|
| P value | r | P value | r | P value | r | |
| Biochemical index | ||||||
| Scr | 0.051 | 0.174 | <0.001 | −0.306 | <0.001 | 0.310 |
| Albumin | 0.305 | −0.092 | 0.394 | 0.077 | 0.196 | −0.116 |
| WBC | 0.106 | 0.145 | 0.048 | −0.176 | 0.024 | 0.201 |
| CRP | 0.002 | 0.273 | 0.001 | −0.289 | <0.001 | 0.335 |
| PCT | 0.008 | 0.235 | 0.065 | −0.165 | 0.009 | 0.231 |
| Disease severity | ||||||
| APACHE II score | <0.001 | 0.291 | 0.001 | −0.292 | <0.001 | 0.354 |
| SOFA score | <0.001 | 0.375 | 0.009 | −0.233 | <0.001 | 0.365 |
| Inflammatory cytokine | ||||||
| TNF‐α | 0.001 | 0.283 | 0.002 | −0.273 | <0.001 | 0.318 |
| IL‐6 | 0.243 | 0.105 | 0.002 | −0.276 | 0.010 | 0.229 |
| IL‐8 | 0.001 | 0.294 | 0.003 | −0.262 | <0.001 | 0.323 |
| IL‐17 | 0.100 | 0.147 | <0.001 | −0.312 | 0.004 | 0.252 |
Correlations were determined by Spearman's rank test. P value < 0.05 was considered significant.
Abbreviations: APACHE II, acute physiology and chronic health evaluation II; CRP, C‐reactive protein; IL, interleukin; PCT, procalcitonin; r, correlation coefficient; Scr, serum creatinine; SOFA, sequential organ failure assessment; TNF‐α, tumor necrosis factor‐α; WBC, white blood cell.
3.4. Predictive value of lnc‐ANRIL/miR‐125a axis for 28‐day death in sepsis patients
The lnc‐ANRIL (P < 0.001; Figure 3A) and lnc‐ANRIL/miR‐125a (P < 0.001; Figure 3C) expressions were upregulated, while the miR‐125a expression (P < 0.001; Figure 3B) in plasma was decreased in deaths than those in survivors. And ROC curve analyses illuminated that lnc‐ANRIL and miR‐125a were capable of differentiating deaths from survivors with AUCs of 0.756 (95% CI: 0.667‐0.843) and 0.745 (95%CI: 0.641‐0.848), and lnc‐ANRIL/miR‐125a axis also presented with a good value in discriminating deaths from survivors with a numerically elevated AUC value of 0.785 (95%CI: 0.703‐0.868; Figure 3D). In addition, univariate logistic regression model analysis disclosed that high lnc‐ANRIL/miR‐125a axis (P < 0.001), Scr (P = 0.016), PCT (P = 0.023), APACHE II score (P = 0.031), SOFA score (P < 0.001), IL‐8 (P < 0.001), and IL‐17 (P = 0.003) were predictive factors for elevated 28‐day mortality (Table 3). Then, all the factors were included in the multivariate logistic regression model analysis, which displayed that high lnc‐ANRIL/miR‐125a axis (P = 0.002) was an independent predictive factor for 28‐day mortality, and Scr (P = 0.010), PCT (P = 0.041), SOFA score (P = 0.048) as well as IL‐8 (P < 0.001) were also independent predictive factors for increased 28‐day mortality.
Figure 3.
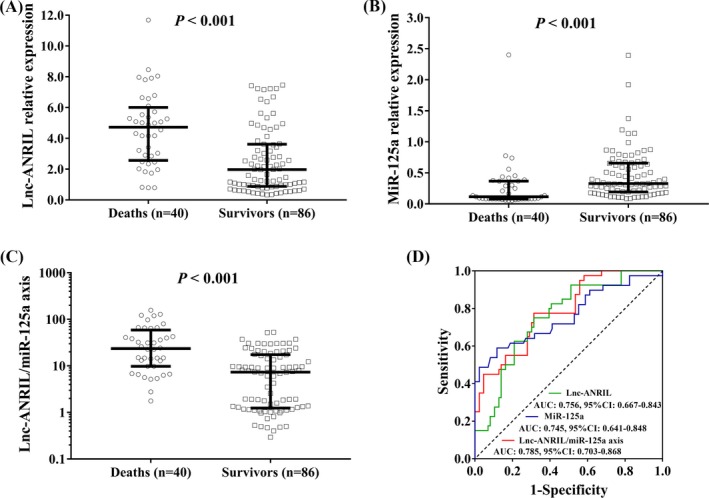
The predictive value of lnc‐ANRIL/miR‐125a axis for 28‐day mortality. Plasma lnc‐ANRIL (A) and lnc‐ANRIL/miR‐125a axis (C) levels were upregulated, while miR‐125a expression (B) was decreased in plasma in deaths than those in survivors. ROC curve analyses illuminated that lnc‐ANRIL, miR‐125a, and lncARIL/miR‐125a expressions were capable of differentiating deaths from survivors (D). Comparison between two groups was determined by Wilcoxon rank‐sum test. Variables' diagnostic value for sepsis and predicting value for death were assessed by ROC curve and the derived area AUC P value < 0.05 was considered as significant. ANRIL, antisense noncoding RNA in the INK4 locus; AUC, area under the curve; ROC, receiver operating characteristic
Table 3.
Univariate and multivariate logistic regression model analyses of factors affecting 28‐day mortality
| Items | Univariate logistic regression | Multivariate logistic regression | ||
|---|---|---|---|---|
| P value | OR (95%CI) | P value | OR (95%CI) | |
| lnc‐ANRIL/miR‐125a axis (high vs low) | <0.001 | 5.812 (2.456‐13.754) | 0.002 | 8.946 (2.310‐34.654) |
| Age (≥56 vs < 56 y) | 0.166 | 0.585 (0.274‐1.248) | 0.105 | 0.360 (0.104‐1.238) |
| Gender (male vs female) | 0.888 | 0.945 (0.429‐2.079) | 0.087 | 0.321 (0.088‐1.179) |
| BMI (≥22.5 vs < 22.5 kg/m2) | 0.903 | 0.955 (0.451‐2.021) | 0.755 | 1.227 (0.340‐4.435) |
| Scr (≥1.7 vs < 1.7 mg/dL) | 0.016 | 2.623 (1.195‐5.762) | 0.010 | 9.328 (1.711‐50.839) |
| Albumin (≥24.7 vs < 24.7 g/L) | 0.058 | 0.475 (0.220‐1.025) | 0.373 | 0.523 (0.126‐2.177) |
| WBC (≥18.4 vs < 18.4 × 109/L) | 0.252 | 1.556 (0.730‐3.316) | 0.186 | 0.368 (0.083‐1.620) |
| CRP (≥103.6 vs < 103.6 mg/L) | 0.128 | 1.808 (0.844‐3.872) | 0.397 | 0.487 (0.092‐2.574) |
| PCT (≥14.2 vs < 14.2 ng/mL) | 0.023 | 2.459 (1.130‐5.351) | 0.041 | 2.475 (1.037‐5.907) |
| APACHE II score (≥14 vs < 14) | 0.031 | 2.346 (1.079‐5.101) | 0.775 | 0.814 (0.197‐3.354) |
| SOFA score (≥5 vs < 5) | <0.001 | 4.818 (2.086‐11.131) | 0.048 | 4.542 (1.014‐20.349) |
| TNF‐α (≥195.0 vs < 195.0 pg/mL) | 0.252 | 1.556 (0.730‐3.316) | 0.702 | 1.175 (0.514‐2.683) |
| IL‐6 (≥61.9 vs < 61.9 pg/mL) | 0.702 | 1.158 (0.546‐2.454) | 0.653 | 0.828 (0.364‐1.884) |
| IL‐8 (≥54.6 vs < 54.6 pg/mL) | <0.001 | 7.097 (2.911‐17.300) | <0.001 | 73.388 (8.843‐609.048) |
| IL‐17 (≥129.4 vs < 129.4 pg/mL) | 0.003 | 3.400 (1.525‐7.578) | 0.111 | 3.569 (0.748‐17.041) |
Continuous variables were divided by the median values. P value < 0.05 was considered significant.
Abbreviations: APACHE II, acute physiology and chronic health evaluation II; BMI, body mass index; CI, confidence interval; CRP, C‐reactive protein; IL, interleukin; OR, odds ratio; PCT, procalcitonin; Scr, serum creatinine; SOFA, sequential organ failure assessment; TNF‐α, tumor necrosis factor‐α; WBC, white blood cell.
3.5. Correlation of lnc‐ANRIL/miR‐125a axis with accumulating survival in sepsis patients
The Kaplan‐Meier curves showed that the accumulating survival was worse in patients with lnc‐ANRIL high expression (P < 0.001; Figure 4A) than that in patients with low lnc‐ANRIL expression, while was better in patients with miR‐125a high expression that that in patients with miR‐125a low expression (P = 0.001; Figure 4B). Most importantly, patients who had high lnc‐ANRIL/miR‐125a axis presented with less satisfactory accumulating survival compared with patients with low lnc‐ANRIL/miR‐125a axis (P < 0.001; Figure 4C). Univariate Cox's proportional hazards model analysis was performed to evaluate the factors for predicting accumulating survival, which showed that high lnc‐ANRIL/miR‐125a axis (P < 0.001), Scr (P = 0.022), PCT (P = 0.018), APACHE II score (P = 0.033), SOFA score (P < 0.001), IL‐8 (P < 0.001), and IL‐17 (P = 0.003) were factors for predicting worse accumulating survival in sepsis patients (Table 4). Afterward, all the factors were analyzed by multivariate Cox's proportional hazards model analysis, which revealed that high lnc‐ANRIL/miR‐125a axis (P = 0.013) independently predicted worse accumulating survival in sepsis patients, and other independent pejorative factors for accumulating survival included increased levels of Scr (P = 0.011), PCT (P = 0.021) and IL‐8 (P < 0.001).
Figure 4.
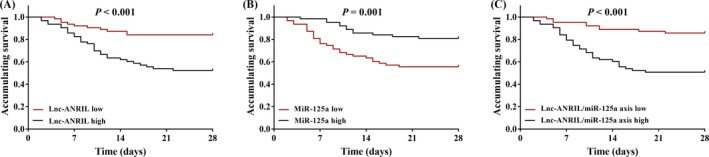
Accumulating survival of sepsis patients. Sepsis patients with high lnc‐ANRIL expression (A), low miR‐125a expression (B), and elevated lnc‐ANRIL/miR‐125a axis (C) in plasma presented with worse accumulating survival. Accumulating survival was illustrated using Kaplan‐Meier curve, and the difference of which was determined by the log‐rank test. P value < 0.05 was considered as significant. ANRIL, antisense noncoding RNA in the INK4 locus
Table 4.
Univariate and multivariate Cox's proportional hazards model analyses of factors affecting accumulating survival
| Items | Univariate Cox's regression | Multivariate Cox's regression | ||
|---|---|---|---|---|
| P value | HR (95%CI) | P value | HR (95%CI) | |
| lnc‐ANRIL/miR‐125a axis (high vs low) | <0.001 | 4.365 (2.075‐9.185) | 0.013 | 3.050 (1.269‐7.331) |
| Age (≥56 vs <56 y) | 0.153 | 0.633 (0.338‐1.185) | 0.176 | 0.648 (0.346‐1.214) |
| Gender (male vs female) | 0.904 | 0.961 (0.502‐1.840) | 0.218 | 0.589 (0.254‐1.367) |
| BMI (≥22.5 vs <22.5 kg/m2) | 0.920 | 0.969 (0.521‐1.801) | 0.326 | 1.523 (0.658‐3.521) |
| Scr (≥1.7 vs <1.7 mg/dL) | 0.022 | 2.166 (1.117‐4.200) | 0.011 | 4.343 (1.402‐13.451) |
| Albumin (≥24.7 vs < 24.7 g/L) | 0.070 | 0.552 (0.291‐1.049) | 0.101 | 0.463 (0.184‐1.163) |
| WBC (≥18.4 vs <18.4 × 109/L) | 0.274 | 1.419 (0.758‐2.657) | 0.119 | 0.486 (0.196‐1.205) |
| CRP (≥103.6 vs <103.6 mg/L) | 0.114 | 1.665 (0.884‐3.135) | 0.931 | 1.040 (0.422‐2.566) |
| PCT (≥14.2 vs <14.2 ng/mL) | 0.018 | 2.187 (1.141‐4.190) | 0.021 | 2.158 (1.126‐4.137) |
| APACHE II score (≥14 vs <14) | 0.033 | 2.032 (1.060‐3.892) | 0.745 | 0.850 (0.320‐2.261) |
| SOFA score (≥5 vs <5) | <0.001 | 3.826 (1.868‐7.836) | 0.072 | 2.607 (0.919‐7.400) |
| TNF‐α (≥195.0 vs <195.0 pg/mL) | 0.221 | 1.479 (0.790‐2.769) | 0.126 | 0.524 (0.229‐1.199) |
| IL‐6 (≥61.9 vs <61.9 pg/mL) | 0.559 | 1.203 (0.647‐2.238) | 0.213 | 0.612 (0.283‐1.324) |
| IL‐8 (≥54.6 vs <54.6 pg/mL) | <0.001 | 5.279 (2.428‐11.478) | <0.001 | 9.421 (2.798‐31.727) |
| IL‐17 (≥129.4 vs <129.4 pg/mL) | 0.003 | 2.838 (1.442‐5.584) | 0.076 | 2.336 (0.915‐5.961) |
Continuous variables were divided by the median values. P value < 0.05 was considered significant.
Abbreviations: APACHE II, acute physiology and chronic health evaluation II; BMI, body mass index; CI, confidence interval; CRP, C‐reactive protein; HR, hazard ratio; IL, interleukin; PCT, procalcitonin; Scr, serum creatinine; SOFA, sequential organ failure assessment; TNF‐α, tumor necrosis factor‐α; WBC, white blood cell.
4. DISCUSSION
In this study, we found that (a) lnc‐ANRIL/miR‐125a axis had good value for predicting sepsis risk; (b) lnc‐ANRIL/miR‐125a axis was positively correlated with disease severity and pro‐inflammatory cytokine levels in sepsis patients; and (c) high lnc‐ANRIL/miR‐125a axis was an independent predictive factor for 28‐day mortality and worse accumulating survival in sepsis patients.
Lnc‐ANRIL, locating at the CDKN2A/B locus, is an ncRNA responsible for multiple biological processes, such as regulating tumor cell proliferation, apoptosis, senescence, and aging.23, 24 Since its first discovery in melanoma, lnc‐ANRIL has been extensively studied, despite the fact that its role in oncology, the association of lnc‐ANRIL with inflammation and immunity has also been found recently.25 An in vitro and in vivo experiment shows that the inflammatory response mediated by lnc‐ANRIL markedly decreases the protective effect of rhein in rat models of uric acid nephropathy.13 Another experiment discloses that downregulating lnc‐ANRIL represses the progression of ulcerative colitis, an inflammatory disease, via mediating the miR‐323b‐5p/TLR4/MyD88/NF‐κB signaling pathway.26 In addition, an experiment that is also conducted in rat models of uric acid nephropathy reveals that lnc‐ANRIL enhances the activation of the NLRP3 inflammasome by regulating the miR‐122‐5p/BRCC3 axis.27 With respect to the role of lnc‐ANRIL in immunity, another critical regulator in sepsis, there have been a few studies illuminating that lnc‐ANRIL might involve in immune disorder related diseases. A study elucidates that the genetic variations of lnc‐ANRIL, which are CCGG and TAAA haplotypes, have a protective effect against multiple sclerosis (MS) in MS patients.14 However, the mechanistic process of lnc‐ANRIL in regulating immune disorder is still not evaluated, more experimental studies are needed in the future. These previous findings of lnc‐ANRIL suggest that lnc‐ANRIL might be a mediator of inflammatory and immune responses in various diseases. Thus, we hypothesized that it might be involved in the development of sepsis which is featured by greatly increased immune responses and inflammation, and we observed that lnc‐ANRIL expression in plasma had good predictive value for sepsis risk and was positively correlated with severity and pro‐inflammatory cytokines levels, which was probably derived from that lnc‐ANRIL could promote the progression of inflammation in sepsis through enhancing inflammatory responses or inflammasome expressions through mediating multiple signaling pathways, such as increasing NLRP3 inflammasome by regulating miR‐122‐5p/BRCC3 axis.13, 25, 26, 27
MiRNAs, another diversified class of ncRNAs, may play critical roles in sepsis development and progression; thus, studies emerge in large numbers to evaluate the roles of miRNAs in the etiology of sepsis. miR‐125a belongs to the miR‐125 family, and the family includes miR‐125a, miR‐125b1 and miR‐126b2.28 An experiment reveals that miR‐125a negative regulates NF‐kB pathway via directly targeting TRAF6, reducing the inflammatory cytokines expressions in mycobacterium tuberculosis infection.19 It is also reported that miR‐125a mimic ameliorates the effect of IL‐6 on promoting the neutrophil chemoattractant expression both in vitro and in vivo in lung inflammation.17 Another experiment reports that miR‐125a targets effector programs and subsequently stabilizes the immune homeostasis mediated by Tregs.29 These mechanistic experiments indicate that miR‐125a is a crucial regulator in inflammation and immune responses, which might explain the results in our study that miR‐125a plasma expression could predict sepsis risk, and was negatively correlated with severity and pro‐inflammatory cytokines levels in sepsis patients. In addition, there is a report showing that U6 might not be an appropriate internal reference for miRNAs detection; however, for circulating miR‐125a expression detection, there are still mounting studies using U6 as an internal reference.12, 30, 31, 32
There are several reports illuminating that miR‐125a is a target of lnc‐ANRIL; thus, since that both lnc‐ANRIL and miR‐125a are implicated in the regulation of inflammation and immunity, we presumed that lnc‐ANRIL might participate in sepsis pathogenesis via modulating miR‐125a.21, 33, 34 Therefore, we conducted the present study and found that plasma lnc‐ANRIL/miR‐125a axis had great value for predicting risk of sepsis, and the AUC of lnc‐ANRIL/miR‐125a axis for predicting the sepsis risk was increased compared with lnc‐ANRIL or miR‐125a alone, indicating lnc‐ANRIL/miR‐125a axis expression is more efficient in predicting the sepsis risk. And lnc‐ANRIL/miR‐125a was positively associated with the disease severity and pro‐inflammatory cytokines level. In addition, it was an independent predictive factor for 28‐day mortality and poor accumulating survival. Our study indicated that plasma lnc‐ANRIL/miR‐125a axis has the potential to be a biomarker for risk, severity, and prognosis of sepsis, which could contribute to the optimization of diagnosis, disease surveillance, and timely intervention of sepsis patients.
The limitations of the study were as follows (a) the sample size was relatively small, which might reduce the statistical power in this study; (b) we only evaluated the short‐term outcome of patients in this study; thus, the value of lnc‐ANRIL/miR‐125a axis in predicting long‐term outcome in sepsis patients was not assessed in this study; (c) the sepsis patients in our study mostly had moderate‐to‐severe sepsis; thus, the role of lnc‐ANRIL/miR‐125a axis in patients with less severe sepsis might not be assessed in our study; (d) the molecular mechanism of the lnc‐ANRIL/miR‐125a axis in regulating sepsis development and progression was not investigated in our study. Thus, a study with larger sample size, longer follow‐up duration, inclusion of patients with less severe sepsis, and molecular experiments should be conducted in the future.
In conclusion, circulating lnc‐ANRIL/miR‐125a axis was upregulated and could serve as a biomarker for severity, inflammation, and prognosis in sepsis patients.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
ACKNOWLEDGMENTS
None.
Gui F, Peng H, Liu Y. Elevated circulating lnc‐ANRIL/miR‐125a axis level predicts higher risk, more severe disease condition, and worse prognosis of sepsis. J Clin Lab Anal. 2019;33:e22917 10.1002/jcla.22917
Feng Gui and Huan Peng contributed equally to this work.
REFERENCES
- 1. Adhikari NK, Fowler RA, Bhagwanjee S, Rubenfeld GD. Critical care and the global burden of critical illness in adults. Lancet. 2010;376(9749):1339‐1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet. 2018;392(10141):75‐87. [DOI] [PubMed] [Google Scholar]
- 3. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis‐3). JAMA. 2016;315(8):801‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bloos F, Reinhart K. Rapid diagnosis of sepsis. Virulence. 2014;5(1):154‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care. 2010;14(1):R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holdt LM, Stahringer A, Sass K, et al. Circular non‐coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat Commun. 2016;7(1):12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brudecki L, Ferguson DA, McCall CE, El Gazzar M. MicroRNA‐146a and RBM4 form a negative feed‐forward loop that disrupts cytokine mRNA translation following TLR4 responses in human THP‐1 monocytes. Immunol Cell Biol. 2013;91(8):532‐540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Precone V, Stornaiuolo G, Amato A, Brancaccio G, Nardiello S, Gaeta GB. Different changes in mitochondrial apoptotic pathway in lymphocytes and granulocytes in cirrhotic patients with sepsis. Liver Int. 2013;33(6):834‐842. [DOI] [PubMed] [Google Scholar]
- 10. Wang K, Long BO, Liu F, et al. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR‐223. Eur Heart J. 2016;37(33):2602‐2611. [DOI] [PubMed] [Google Scholar]
- 11. Zhang T‐N, Li DA, Xia J, et al. Non‐coding RNA: a potential biomarker and therapeutic target for sepsis. Oncotarget. 2017;8(53):91765‐91778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu Y, Shao B. Circulating lncRNA IFNG‐AS1 expression correlates with increased disease risk, higher disease severity and elevated inflammation in patients with coronary artery disease. J Clin Lab Anal. 2018;32(7):e22452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hu J, Wang D, Wu H, Yang Z, Yang N, Dong J. Long non‐coding RNA ANRIL‐mediated inflammation response is involved in protective effect of rhein in uric acid nephropathy rats. Cell Biosci. 2019;9(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rezazadeh M, Gharesouran J, Moradi M, et al. Association study of ANRIL genetic variants and multiple sclerosis. J Mol Neurosci. 2018;65(1):54‐59. [DOI] [PubMed] [Google Scholar]
- 15. Essandoh K, Li Y, Huo J, Fan GC. MiRNA‐mediated macrophage polarization and its potential role in the regulation of inflammatory response. Shock. 2016;46(2):122‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Monk CE, Hutvagner G, Arthur JS. Regulation of miRNA transcription in macrophages in response to Candida albicans. PLoS ONE. 2010;5(10):e13669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith S, Wu PW, Seo JJ, et al. IL‐16/miR‐125a axis controls neutrophil recruitment in pristane‐induced lung inflammation. JCI Insight. 2018;3(15):pii 120798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hsu A‐Y, Dua K, Starkey MR, et al. MicroRNA‐125a and ‐b inhibit A20 and MAVS to promote inflammation and impair antiviral response in COPD. JCI Insight. 2017;2(7):e90443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Niu W, Sun B, Li M, Cui J, Huang J, Zhang L. TLR‐4/microRNA‐125a/NF‐kappaB signaling modulates the immune response to Mycobacterium tuberculosis infection. Cell Cycle. 2018;17(15):1931‐1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li R, Yin F, Guo YY, Zhao KC, Ruan Q, Qi YM. Knockdown of ANRIL aggravates H 2 O 2 ‐induced injury in PC‐12 cells by targeting microRNA‐125a. Biomed Pharmacother. 2017;92:952–961. [DOI] [PubMed] [Google Scholar]
- 21. Hu X, Jiang H, Jiang X. Downregulation of lncRNA ANRIL inhibits proliferation, induces apoptosis, and enhances radiosensitivity in nasopharyngeal carcinoma cells through regulating miR‐125a. Cancer Biol Ther. 2017;18(5):331‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Intensive Care Med. 2003;29(4):530‐538. [DOI] [PubMed] [Google Scholar]
- 23. Matheu A, Maraver A, Collado M, et al. Anti‐aging activity of the Ink4/Arf locus. Aging Cell. 2009;8(2):152‐161. [DOI] [PubMed] [Google Scholar]
- 24. Cánepa ET, Scassa ME, Ceruti JM, et al. INK4 proteins, a family of mammalian CDK inhibitors with novel biological functions. IUBMB Life. 2007;59(7):419‐426. [DOI] [PubMed] [Google Scholar]
- 25. Pasmant E, Laurendeau I, Heron D, Vidaud M, Vidaud D, Bieche I. Characterization of a germ‐line deletion, including the entire INK4/ARF locus, in a melanoma‐neural system tumor family: identification of ANRIL, an antisense noncoding RNA whose expression coclusters with ARF. Cancer Res. 2007;67(8):3963‐3969. [DOI] [PubMed] [Google Scholar]
- 26. Qiao C, Yang L, Wan J, et al. Long noncoding RNA ANRIL contributes to the development of ulcerative colitis by miR‐323b‐5p/TLR4/MyD88/NF‐kappaB pathway. Biochem Biophys Res Commun. 2019;508(1):217‐224. [DOI] [PubMed] [Google Scholar]
- 27. Hu J, Wu H, Wang D, Yang Z, Dong J. LncRNA ANRIL promotes NLRP3 inflammasome activation in uric acid nephropathy through miR‐122‐5p/BRCC3 axis. Biochimie. 2019;157:102–110. [DOI] [PubMed] [Google Scholar]
- 28. Potenza N, Russo A. Biogenesis, evolution and functional targets of microRNA‐125a. Mol Genet Genomics. 2013;288(9):381‐389. [DOI] [PubMed] [Google Scholar]
- 29. Pan W, Zhu S, Dai D, et al. MiR‐125a targets effector programs to stabilize Treg‐mediated immune homeostasis. Nat Commun. 2015;6(1):7096. [DOI] [PubMed] [Google Scholar]
- 30. Chen J, Ouyang H, An X, Liu S. miR‐125a is upregulated in cancer stem‐like cells derived from TW01 and is responsible for maintaining stemness by inhibiting p53. Oncol Lett. 2019;17(1):87‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu H‐L, Nie Z‐Q, Lu Y, et al. Circulating miR‐125b but not miR‐125a correlates with acute exacerbations of chronic obstructive pulmonary disease and the expressions of inflammatory cytokines. Medicine. 2017;96(51):e9059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen D, Huang X, Lu S, et al. miRNA‐125a modulates autophagy of thyroiditis through PI3K/Akt/mTOR signaling pathway. Exp Ther Med. 2019;17(4):2465‐2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chai L, Yuan Y, Chen C, Zhou J, Wu Y. The role of long non‐coding RNA ANRIL in the carcinogenesis of oral cancer by targeting miR‐125a. Biomed Pharmacother. 2018;103:38–45. [DOI] [PubMed] [Google Scholar]
- 34. Zhang LM, Ju HY, Wu YT, et al. Long non‐coding RNA ANRIL promotes tumorgenesis through regulation of FGFR1 expression by sponging miR‐125a‐3p in head and neck squamous cell carcinoma. Am J Cancer Res. 2018;8(11):2296‐2310. [PMC free article] [PubMed] [Google Scholar]


