Significance
Captive rearing and release of monarch butterflies is a cultural phenomenon in the United States, where commercial breeders sell monarchs for release by school children and hobbyists raise wild monarchs in an effort to boost dwindling numbers. Our research shows that the captive breeding of monarchs disrupts critical aspects of their migratory behavior. The results are important because they reveal that different components of the migratory syndrome are easily decoupled and that migratory behavior is remarkably sensitive to genetic and environmental change. These results are relevant to conservation efforts, especially as the US Fish and Wildlife Service considers whether to list the North American monarch as a threatened species under the US Endangered Species Act.
Keywords: Danaus plexippus, captive breeding, behavior, migration, population genetics
Abstract
The annual migration of the monarch butterfly Danaus plexippus is in peril. In an effort to aid population recovery, monarch enthusiasts across North America participate in a variety of conservation efforts, including captive rearing and release of monarch butterflies throughout the summer and autumn. However, the impact of captive breeding on monarchs remains an open question. Here, we show that captive breeding, both commercially and by summertime hobbyists, causes migratory behavior to be lost. Monarchs acquired commercially failed to orient south when reared outdoors in the autumn, unlike wild-caught North American monarchs, yet they did enter reproductive diapause. The commercial population was genetically highly divergent from wild-caught North American monarchs and had rounder forewings, similar to monarchs from nonmigratory populations. Furthermore, rearing wild-caught monarchs in an indoor environment mimicking natural migration-inducing conditions failed to elicit southward flight orientation. In fact, merely eclosing indoors after an otherwise complete lifecycle outdoors was enough to disrupt southern orientation. Our results provide a window into the complexity—and remarkable fragility—of migration.
The monarch butterfly Danaus plexippus is famous for its annual mass migration across North America (1, 2). Unfortunately, the number of overwintering monarchs in Mexico has declined drastically over the past 25 y (3, 4). Out of concern that the monarch migration may go extinct in the foreseeable future (5), the US Fish and Wildlife Service is currently considering whether to list the monarch butterfly as a threatened species under the US Endangered Species Act (6). While there is some disagreement about primary drivers of monarch population decline (7–16), the public maintains a keen interest in monarch conservation and undertakes a variety of activities every year to aid them, including reporting sightings online, planting milkweed, creating migratory waystations, and even raising monarchs for release.
However, captive rearing of monarchs is a contentious practice. Summertime hobbyists raise monarchs in their homes throughout the summer and autumn and then release them, hoping that they or their offspring will fly south to Mexico and ultimately contribute to population recovery. Conservation groups and scientists have expressed concern that captive rearing may result in higher parasite loads and even adaptation to captive conditions (17–21). Formal captive breeding programs, which are sometimes implemented to aid recovery of threatened or endangered species, do not exist for the monarch butterfly, but there are multiple commercial companies that breed monarchs year-round and sell them for release. These commercial monarchs are raised and released by school children across the United States, again with the belief that they will fly to the overwintering ground. These monarchs are also released at special events like weddings and monarch-themed fall festivals. However, the impact of captive breeding on monarch migration biology has not been investigated. In this study, we explored whether monarch breeding by commercial facilities and hobbyists affects migration phenotypes and genetics of captive-reared monarchs.
Results and Discussion
To investigate the migratory status of commercially bred monarchs, we reared both commercially sourced and wild-caught North American (NA) monarchs in a common garden experiment. We ordered adult monarchs from a commercial breeder and caught adult wild NA monarchs in July 2016. We raised the offspring of both groups over two successive generations, summer and autumn, in outdoor insectaries in Chicago, IL. Our experiment focused on comparing the descendants of commercial and wild-caught NA monarchs and of crosses between the two groups, raised at the same time in the same outdoor conditions. The monarch migratory syndrome is a multifaceted phenotype, encompassing behavioral, physiological, and anatomical traits. We assessed all three of these components by measuring flight orientation, reproductive status, and wing shape.
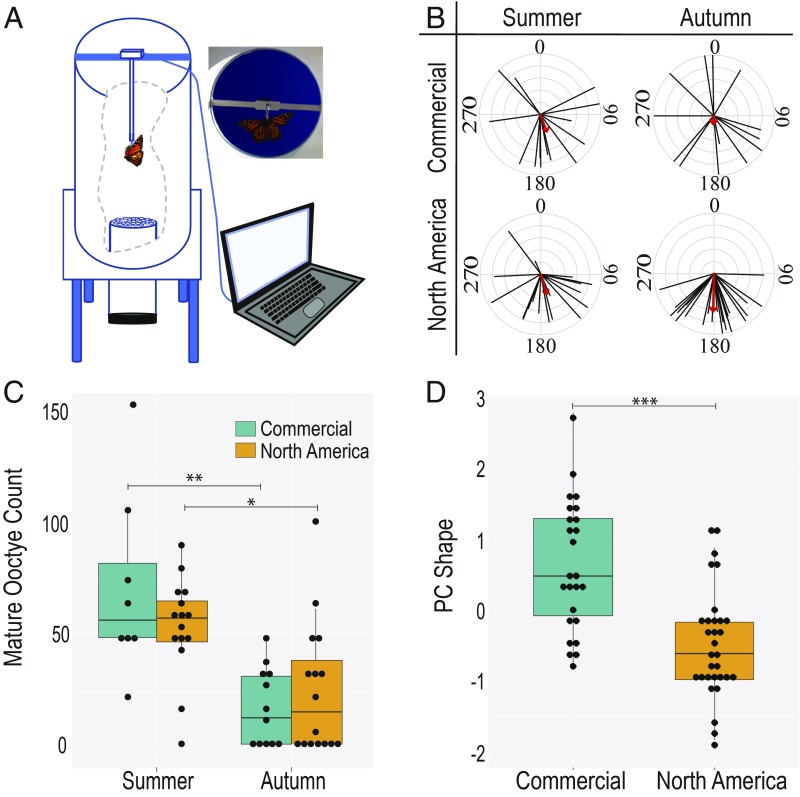
To measure orientation behavior, we tested monarchs in a monarch flight simulator (Fig. 1A) (22). Previous work using the simulator has shown that summer-generation monarchs do not have a group direction, whereas autumn-generation monarchs fly south (22–26). We calculated the group mean vector (0° to 359°), weighted by the strength of each individual’s vector (0 to 1), as well as the group vector strength. We then used the Rayleigh test to determine whether each group was directional. NA monarchs behaved as expected. NA monarchs that emerged in October flew directionally south (Fig. 1B; σ = 181°, n = 25, r = 0.65, Rayleigh test, z score = 10.65, P < 0.001), and those that emerged in August flew weakly south (Fig. 1B; σ = 161°, n = 19, r = 0.37, Rayleigh test, z score = 2.6, 0.05 < P < 0.1). Surprisingly, commercial monarchs that were raised side-by-side with NA monarchs did not have a mean direction in either late summer or autumn (Fig. 1B; summer σ = 158°, n = 14, r = 0.32, Rayleigh test, z score = 1.43, P > 0.2; autumn σ = 183°, n = 14, r = 0.16, Rayleigh test, z score = 0.36, P > 0.5). Consistent with these results, we found that the distribution of directions between commercial and NA monarchs did not differ in the summer (Wallraff test, Kruskal–Wallis χ2 = 0.0212, P = 0.884), but did in autumn (Wallraff test, Kruskal–Wallis χ2 = 5.763, P = 0.016). Similarly, the distributions of group vector strengths of commercial and NA groups overlapped in the summer, but not in autumn (SI Appendix, Fig. S1A). Additionally, these results suggest that the genetic basis of directional orientation is dominant to nonorientation because our breeding design involved comparing autumn-generation local monarchs that were actually female NA × commercial hybrids backcrossed to pure NA male monarchs (75% NA, 25% commercial genome). All of the hybrid NA × commercial females had NA mothers, giving them and their offspring a NA mitochondrial genome.
Fig. 1.
Commercial-lineage monarchs do not orient south but enter reproductive arrest. (A) A representation of the flight simulator. The rotary encoder captures the orientation data while video is recorded. Laminar airflow is generated by the fan and vertical drinking straws. (B) Orientation plots of commercial versus NA monarchs raised in the summer and autumn. Each black line indicates the mean direction (0° to 359°) of an individual butterfly, and the length of the line represents the strength of that direction (0 to 1). The red arrow indicates the mean direction of the group, and the length of the arrow indicates the strength of the group direction. 0° is north. (C) The number of mature oocytes of each female who completed a flight test. Both commercial (teal) and NA (orange) females enter reproductive arrest in the autumn as evidenced by their lower oocyte counts. The number of asterisks denote statistical significance level: *P < 0.05, **P < 0.01, and ***P < 0.001. (D) Comparison of commercial (teal) and NA (orange) forewing shape. Principal component (PC) Shape is the first PC of the PCA and explains 55.53% of the variation in shape based on geometric morphometric analysis of 13 landmarks on the wing.
We next looked at whether commercial monarchs enter diapause, which keeps NA monarchs in reproductive arrest during their migration, by counting the number of mature oocytes in each female that flew in the flight orientation assay. We found that commercial monarchs did enter reproductive arrest like NA monarchs. Commercial monarchs averaged 70.4 ± 14.66 (SE) oocytes (n = 8) in August, which decreased to just 16.8 ± 5 (SE) (n = 12) in October (Fig. 1C). The NA individuals averaged 53 ± 5.72 (SE) oocytes (n = 15) in August and decreased to 24.3 ± 7.45 (SE) (n = 16) in October (Fig. 1C). The number of mature oocytes decreased in both populations from August to October (Mann–Whitney U test; commercial, P = 0.003; NA, P = 0.013). There was no difference between commercial and NA oocyte counts in either season (Mann–Whitney U test, P > 0.5). In contrast to previous results showing that all outdoor-reared females emerging in September had no mature oocytes (27), only 38% of females had no mature oocytes in the autumn. As a whole, these results demonstrate that components of the migratory syndrome are easily decoupled and that reproductive diapause cannot be used as a proxy for migratory behavior.
Using geometric morphometrics, we compared wing shape and size, which are known to differ between migratory NA monarchs and nonmigratory populations from other locations (28). We found that commercial monarchs had rounder forewings compared with NA monarchs (Mann–Whitney U test, P < 0.001; Fig. 1D and SI Appendix, Fig. S2A)—differences similar to those between natural migratory and nonmigratory populations (28). We also found that forewing shape is sexually dimorphic in both commercial and NA monarchs, with males having rounder forewings than females (SI Appendix, Fig. S3). Additionally, commercial monarchs may have smaller forewings than NA monarchs (Mann–Whitney U test, P = 0.054; SI Appendix, Fig. S4), again mirroring differences between natural migratory and nonmigratory populations (28).
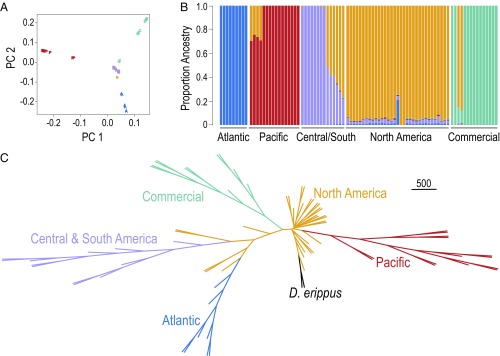
Our results indicate that either (i) long-term captive breeding of this commercial monarch population resulted in the loss of migratory behavior and a change in wing morphology, or (ii) the commercial population in our study was originally founded by or supplemented with monarchs from a nonmigratory population. Over the past hundreds or thousands of years, NA monarchs dispersed out of North America at least three times, once south into Central and South America and the Caribbean, once west across the Pacific Islands and into Australia, and once east into southern Europe and North Africa (29). Each of these dispersal events produced populations that reproduce year-round and do not migrate. It remains unknown whether these populations do not migrate because they have lost the ability or because they do not experience the relevant environmental cues. To determine the ancestry of the commercial population, we generated whole-genome sequencing (WGS) data from 15 commercial specimens, 14 of which successfully completed an autumn flight test, and compared them to a worldwide sample of monarch genomes (29). After filtering, our analysis was based on 4,593,379 single-nucleotide polymorphisms (SNPs) with an overall genotyping rate of 0.995. Principal component analysis (PCA) showed that the commercial lineage did not cluster with any other known monarch population, including North America (29) (Fig. 2A). Using a pruned dataset of 1 million variants, we inferred population subdivision and admixture using Frappe, version 1.1 (30). Consistent with previous work, we found that samples collected from around the world represent at least four distinct populations: North America, Central/South America, Pacific, and Atlantic (29). The commercial individuals represent a distinct and previously unknown population of monarchs (Fig. 2B). We also found evidence that the commercial breeder does introduce NA genetic variation into their captive population, as two of the 15 commercial samples shared ancestry with NA monarchs (Fig. 2B). However, this supplementing of genetic variation does not appear to have a lasting impact on the commercial population.
Fig. 2.
Commercial monarchs represent a distinct population of monarch butterflies derived from NA monarchs. (A) PCA of SNPs. Principle component 1 (PC1) accounts for 12.3% of the variation in the data, and PC2 accounts for 10.7%. (B) Analysis of SNP structure using Frappe. Each bar represents a single individual, and the colors represent the proportion of ancestry for five populations. (C) Neighbor-joining consensus tree based on SNP data. (Scale bar is equivalent to 500 bootstraps.)
Subsequent phylogenetic analyses indicate that the commercial monarch population was originally derived from North America and there has been no appreciable gene flow into the commercial population from nonmigratory populations. For instance, our phylogenetic analysis recovered the signatures of independent dispersal events out of North America in the founding of worldwide monarch populations, with the addition of a fourth independent event leading to the origin of the commercial population (Fig. 2C). Furthermore, analysis with TreeMix (31) found no evidence of gene flow between the commercial population and any nonmigratory population (SI Appendix, Fig. S5). Consistent with the inferred NA ancestry of the commercial population, we found that the commercial samples that we sequenced were fixed for the NA haplotype at a migration-associated collagen gene, suggesting there is more than one way to become nonmigratory (29). The population genetic consequences of commercial rearing appear to mirror natural dispersal; the commercial lineage, similar to the Atlantic and Pacific populations, was genetically differentiated from NA monarchs and had reduced nucleotide diversity (π) (SI Appendix, Table S1).
We do not know what effect the introduction of nonorienting monarchs might have on the wild NA population or whether these results apply more generally to commercial monarch breeding. However, our results indicate that at least one group of commercially bred monarchs are much less likely to migrate than wild NA monarchs. Nonorienting monarchs released in the autumn are unlikely to migrate successfully and will not contribute to monarch population recovery or to the gene pool. However, nonorienting monarchs released in the summer could mate with wild NA individuals, leading to the introduction of nonmigratory variation that may not be purged. We suspect that without the strong annual selective pressure of migration, migration-associated traits can be lost in captivity.
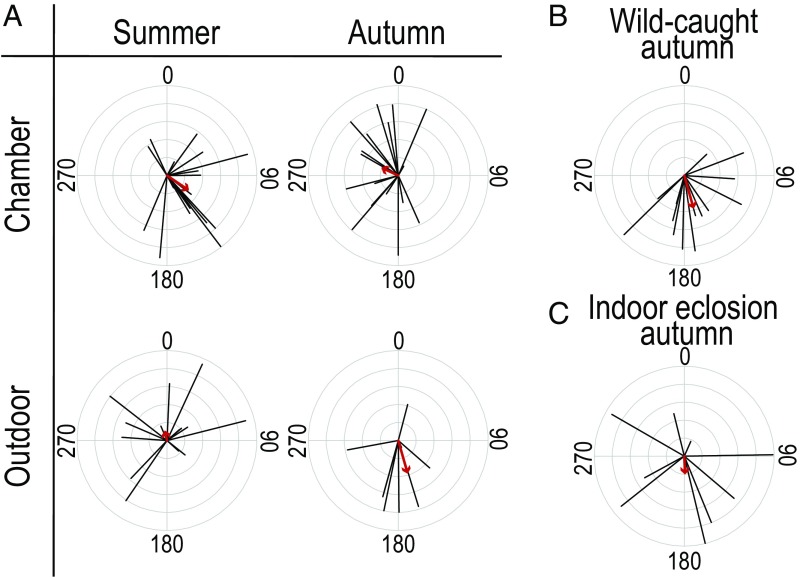
Unlike commercial breeders, hobbyist breeders tend to collect wild eggs throughout the spring and summer and rear them for immediate release or for a few generations during the summer and autumn. When released, autumn-generation butterflies are expected to fly south and experience the same selection pressures as wild individuals. However, we do not know whether rearing a monarch butterfly indoors, where natural environmental cues (temperature, light, etc.) may be absent, affects the induction of migratory behavior. To determine whether indoor captive rearing affects migration, we reared NA monarchs indoors in both an autumnlike (18 °C with a 14-h day) and a summerlike (25 °C with a 16-h day) environmental chamber in 2018. We also reared a summer and an autumn generation outdoors and caught wild autumn-generation monarchs as they migrated south through Chicago in mid-September to act as controls. As expected, outdoor-summer and chamber-summer groups did not orient in a specific direction (Fig. 3A; outdoor σ = 348°, r = 0.12, n = 16, Rayleigh test, z score = 0.23, P > 0.5; chamber σ = 124°, r = 0.295, n = 19, Rayleigh test, z score = 1.65, P > 0.1). In contrast, outdoor-reared autumn-generation and wild-caught autumn-generation monarchs showed southern group orientation (Fig. 3A, outdoor σ = 185°, r = 0.4, n = 9, Rayleigh test, z score = 1.44, P > 0.2; Fig. 3B, wild σ = 164°, r = 0.39, n = 14, Rayleigh test, z score = 2.13, P > 0.1). Our sample sizes for these groups were limited but they did have a significant southward direction when combined (autumn positive controls: σ = 172°, n = 23, r = 0.39, Rayleigh test, z score = 3.50, P < 0.05). Unexpectedly, monarchs reared in the autumnlike chamber did not orient south (Fig. 3A; σ = 295°, r = 0.21, n = 17, Rayleigh test, z score = 0.75, P > 0.2). The distributions of individual directions and group vector strengths differed between autumn chamber monarchs and autumn positive controls (Wallraff test, Kruskal–Wallis χ2 = 5.970, P = 0.015; SI Appendix, Fig. S1B).
Fig. 3.
NA monarchs reared in environmental chambers do not orient south. (A) Orientation plots of NA monarchs raised in a summerlike and an autumnlike chamber and NA monarchs raised outdoors in both a summer and an autumn generation. Each black line indicates the mean direction (0° to 359°) of an individual butterfly, and the length of the line represents the strength of that direction (0 to 1). The red arrow indicates the mean direction of the group, and the length of the arrow indicates the strength of the group direction. 0° is north. (B) Orientation plot of wild NA monarchs caught along their migration route through Chicago, IL. (C) Orientation plot of NA monarchs raised outdoors but brought indoors for eclosion.
Because reproductive diapause is also an important component of the migratory syndrome, we counted mature oocytes in our chamber- and outdoor-reared monarchs. As expected, the autumn females reared outdoors averaged 35.6 ± 11.7 (SE) mature oocytes (n = 7), a marked decrease from those reared in the summer, which averaged 77.2 ± 7 (SE) (n = 9) (SI Appendix, Fig. S6). Unlike the outdoor-reared group, the autumnlike chamber females did not have lower egg counts compared with the summerlike chamber females, averaging 60.4 ± 16.5 (SE) (n = 8) oocytes and 69.9 ± 10.8 (SE) (n = 13), respectively (SI Appendix, Fig. S6). Although some autumn chamber-reared individuals entered diapause, the cool temperature and early-autumn day-length conditions were not sufficient to induce diapause in the entire group, suggesting a missing environmental cue.
We do not know what specifically about the indoor environment prevents the development of migration behavior. Perhaps there are critical developmental periods or environmental conditions that prime monarchs to develop as migratory individuals. We do, however, have one additional observation that illustrates the fragility of migratory orientation behavior. On October 24, 2016, we moved a number of outdoor-reared NA pupae indoors to an autumnlike chamber kept at 21 °C and with an 11-h day (0700 to 1800 hours) to mimic the outdoor environment. The nine individuals who flew in the simulator emerged on either day 3 or 4 after being brought indoors. Even though these individuals spent the vast majority of their development outdoors during the autumn, they did not all orient toward the south like their completely outdoor-reared siblings (Fig. 3C; indoor eclosion NA σ = 177°, r = 0.21, n = 9, Rayleigh test, z score = 0.40, P > 0.5). The indoor eclosion and the autumn positive control groups differed in the distribution of their individual directions (Wallraff test, Kruskal–Wallis χ2 = 7.574, P = 0.006) and group vector strengths (SI Appendix, Fig. S1C). These results suggest that brief exposure to unnatural conditions—even late in development—may be enough to disrupt flight orientation behavior in some monarchs. However, given the small sample size, this result merits further attention in the future.
Our results have a number of practical implications. First, captive-bred monarchs that are reared year-round could potentially lose flight orientation behavior, which would seriously impact their ability to migrate. That being said, we assessed only one commercially bred lineage, and there is evidence that other commercially bred monarchs do migrate. Recently, 720 monarchs that were raised by a different commercial breeder were tagged and released in San Antonio, TX, of which five were recovered at overwintering sites in Mexico (32). We do not know if different husbandry practices affect whether a captive population is likely to lose migration behavior or if some proportion of all commercial monarchs have the potential to orient and migrate successfully. Additional flight testing may reveal that a percentage of the commercially bred monarchs orient correctly; however, as a group, these commercial monarchs are not directional.
In terms of seasonal rearing by summer hobbyists and school groups, we would argue that the practice of raising monarchs in this setting is net positive, especially in the link it creates between people and their natural environment. The fact that so many school-age children raise monarchs is probably one of the reasons the insect is so popular and why the public directly participates in conservation efforts on behalf of the species. This practice should absolutely continue, with the added caveats that the butterflies should be locally sourced and then subsequently reared outdoors where they will be exposed to the full spectrum of natural environmental conditions, ensuring that reared monarchs will have the best chance of migrating successfully.
Our results also have important implications for the larger issue of monarch conservation, especially as it relates to potentially listing the monarch as a threatened species. Even though NA monarchs have dispersed and colonized many parts of the world, these populations do not migrate. While globally the species may survive, the spectacular annual migration of monarchs in North America may be nearing an end. This reality has inspired scientists and conservationists to ask about the nature of migration loss in other environments—are nonmigratory populations simply never exposed to the environmental cues that induce migration or have they lost the trait? A recent study demonstrated that a Pacific monarch lineage enters diapause when reared under autumnlike conditions (33), suggesting that nonmigratory monarchs may retain migration-associated adaptations. However, that study did not assess flight orientation. Our results suggest that recurring selective pressure in the form of annual migration is necessary to maintain the entire suite of migration-associated adaptations.
Materials and Methods
Animal Husbandry.
For outdoor-reared monarchs in 2016, we captured summer-generation adult NA monarchs in Chicago and purchased adult monarchs from a commercial breeder in July. However, the number of wild monarchs in the Chicago area was very low in 2016, so we mated some of our NA wild-caught adults to commercially sourced as well as wild-caught monarchs to produce our late-summer generation. The summer-generation monarchs tested in the flight simulator and dissected for mature oocyte counts emerged between August 11 and August 22, 2016. Because we had very few purebred NA monarchs in our summer generation, we crossed the few remaining 100% NA monarchs to the F1 hybrids to create our NA autumn generation (roughly 75% NA to 25% commercial). We mated pure commercial individuals to each other to produce the commercial autumn generation. We reared the autumn generation outdoors, and they emerged between October 10 and October 24, 2016.
In 2018, we captured summer-generation adult NA monarchs in Chicago in June. We collected eggs from adults housed outdoors in early July and reared the offspring in a summerlike or an autumnlike chamber, as well as keeping a group outdoors. We kept the summerlike chamber at 25 °C with a 16-h day and kept the autumnlike chamber at 18 °C with a 14-h day. Summer-chamber adults emerged between July 31 and August 3, 2018. Autumn-chamber adults emerged between August 26 and August 30, 2018. The outdoor summer generation emerged between August 1 and August 3, 2018, whereas the outdoor autumn generation emerged between September 7 and September 19, 2018. Additionally, we caught 24 wild adult monarchs as they migrated through Chicago on September 14, 2018. Details of rearing and care are described in SI Appendix, Supplemental Materials and Methods.
Flight Testing.
We performed flight testing in a monarch flight simulator adapted from Mouritsen and Frost (22) (Fig. 1A), and used methods consistent with previous flight experiments (22–26) when testing. We performed all tests under sunny skies, and a successful test required that an individual fly continuously for 10 min. After each successful test, we froze the sample for future dissection and potential genetic analysis. We calculated the mean vector (σ = 0° to 359°) and vector strength (r = 0 to 1) for each individual. We then calculated a weighted group mean vector and vector strength. We used the Rayleigh test to determine whether the group mean was significantly directional and used the Wallraff test to determine whether the distribution of individual directions differed. To determine whether our groups had significantly different distributions of their group vector strengths, we applied a bootstrapping analysis (SI Appendix, Fig. S1). For detailed descriptions, see SI Appendix, Supplemental Materials and Methods.
Mature Oocyte Counts.
We dissected females who completed a flight test by making a longitudinal cut down the abdomen to remove ovaries and eggs, and then counted the number of mature oocytes (SI Appendix, Supplemental Materials and Methods).
Geometric Morphometrics.
To examine the shape and size of monarch forewings, we performed geometric morphometric analyses using 13 landmarks (SI Appendix, Fig. S2B). To examine shape alone, we applied a generalized Procrustes analysis to exclude effects unrelated to shape, including reflection, position, scale, and orientation. We examined variation in size and shape with PCA (SI Appendix, Supplemental Materials and Methods).
Population Genetics and Phylogenetics.
We extracted DNA from 15 commercial monarchs, generated paired-end 75-bp libraries, and sequenced the libraries on the NextSeq500 Illumina platform. We downloaded WGS data from the National Ceter for Biotechnology Information Sequence Read Archive for 72 monarch and nine outgroup samples collected from around the world (29) (SI Appendix, Table S2). We mapped sequences to the NA monarch reference genome (version 3, repeat masked) (34). We then assigned sample genotypes and called SNPs/variants using the Genome Analysis Toolkit (35). After filtering, we performed a PCA using the remaining 4,593,379 variants. We estimated population identity from two to eight distinct populations (K = 2 to K = 8) using Frappe, version 1.1 (30) (SI Appendix, Fig. S9). We built a neighbor-joining tree with 500 rapid bootstrap replicates using FastMe (36) (Fig. 2C) and used TreeMix to investigate historical relationships and gene flow among monarch populations (31) (SI Appendix, Fig. S5). Details of these analyses are available in SI Appendix, Supplemental Materials and Methods.
Data Availability.
Whole-genome sequences are available at the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/bioproject) under BioProject accession number PRJNA509269. All other data are available in Dataset S1.
Supplementary Material
Acknowledgments
We thank Dr. Andre Green for significant help in raising the butterflies used in the flight simulator experiments and Dr. Patrick Guerra for his instruction on using the monarch flight simulator. This work was funded by the NSF Graduate Research Fellowship Program, NIH Genetics and Regulation Training Grant T32 GM07197, US Fish and Wildlife Service Award F17AC01222, NSF Grant IOS-1452648, and NIH Grant GM108626.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The whole-genome sequences reported in this paper have been deposited in the National Center for Biotechnology Information, https://www.ncbi.nlm.nih.gov/bioproject (BioProject accession no. PRJNA509269).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1904690116/-/DCSupplemental.
References
- 1.Reppert SM, Gegear RJ, Merlin C (2010) Navigational mechanisms of migrating monarch butterflies. Trends Neurosci 33:399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reppert SM, de Roode JC (2018) Demystifying monarch butterfly migration. Curr Biol 28:R1009–R1022. [DOI] [PubMed] [Google Scholar]
- 3.Brower LP, et al. (2012) Decline of monarch butterflies overwintering in Mexico: Is the migratory phenomenon at risk? Insect Conserv Diversity 5:95–100. [Google Scholar]
- 4.Vidal O, Rendón-Salinas E (2014) Dynamics and trends of overwintering colonies of the monarch butterfly in Mexico. Biol Conserv 180:165–175. [Google Scholar]
- 5.Semmens BX, et al. (2016) Quasi-extinction risk and population targets for the Eastern, migratory population of monarch butterflies (Danaus plexippus). Sci Rep 6:23265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Center for Biological Diversity (2014) Petition to protect the monarch butterfly (Danaus plexippus plexippus) under the Endangered Species Act. Available at https://www.biologicaldiversity.org/species/invertebrates/pdfs/Monarch_ESA_Petition.pdf. Accessed December 6, 2018.
- 7.Agrawal AA, Inamine H (2018) Mechanisms behind the monarch’s decline. Science 360:1294–1296. [DOI] [PubMed] [Google Scholar]
- 8.Badgett G, Davis AK (2015) Population trends of monarchs at a northern monitoring site: Analyses of 19 years of fall migration counts at Peninsula Point, MI. Ann Entomol Soc Am 108:700–706. [Google Scholar]
- 9.Boyle JH, Dalgleish HJ, Puzey JR (2019) Monarch butterfly and milkweed declines substantially predate the use of genetically modified crops. Proc Natl Acad Sci USA 116:3006–3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis AK. (2012) Are migratory monarchs really declining in eastern North America? Examining evidence from two fall census programs. Insect Conserv Diversity 5:101–105. [Google Scholar]
- 11.Inamine H, Ellner SP, Springer JP, Agrawal AA (2016) Linking the continental migratory cycle of the monarch butterfly to understand its population decline. Oikos 125:1081–1091. [Google Scholar]
- 12.Pleasants JM, Williams EH, Brower LP, Oberhauser KS, Taylor OR (2016) Conclusion of no decline in summer monarch population not supported. Ann Entomol Soc Am 109:169–171. [Google Scholar]
- 13.Ries L, Taron DJ, Rendón-Salinas E (2015) The disconnect between summer and winter monarch trends for the eastern migratory population: Possible links to differing drivers. Ann Entomol Soc Am 108:691–699. [Google Scholar]
- 14.Saunders SP, Ries L, Oberhauser KS, Thogmartin WE, Zipkin EF (2018) Local and cross-seasonal associations of climate and land use with abundance of monarch butterflies Danaus plexippus. Ecography 41:278–290. [Google Scholar]
- 15.Stenoien C, Nail KR, Oberhauser KS (2015) Habitat Productivity and temporal patterns of monarch butterfly egg densities in the eastern United States. Ann Entomol Soc Am 108:670–679. [Google Scholar]
- 16.Thogmartin WE, et al. (2017) Monarch butterfly population decline in North America: Identifying the threatening processes. R Soc Open Sci 4:170760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis A. (2018) New statement from monarch conservation groups says–For the love of God, stop mass-rearing monarchs in your kitchens! The Science of Monarch Butterflies: A blog about monarchs, written by a monarch scientist for people who love monarchs. Available at http://akdavis6.wixsite.com/monarchscience/single-post/2018/09/11/New-statement-from-monarch-conservation-groups-says—For-the-love-of-God-stop-mass-rearing-monarchs-in-your-kitchens. Accessed December 6, 2018.
- 18.Malcolm SB. (2018) Anthropogenic impacts on mortality and population viability of the monarch butterfly. Annu Rev Entomol 63:277–302. [DOI] [PubMed] [Google Scholar]
- 19.Pelton E. (2018) Keep monarchs wild! Why captive rearing isn’t the way to help monarchs. Xerxes Society for Invertebrate Conservation. Available at https://xerces.org/2018/09/11/keep-monarchs-wild/. Accessed December 6, 2018.
- 20.Journey North (2015) Captive breeding and releasing monarchs. Available at https://journeynorth.org/tm/monarch/conservation_action_release.pdf. Accessed January 3, 2019.
- 21.Monarch Joint Venture (2018) Revised handout. Raising monarchs: Why or why not? Available at https://monarchjointventure.org/news-events/news/revised-handout-raising-monarchs-why-or-why-not. Accessed January 3, 2019.
- 22.Mouritsen H, Frost BJ (2002) Virtual migration in tethered flying monarch butterflies reveals their orientation mechanisms. Proc Natl Acad Sci USA 99:10162–10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Froy O, Gotter AL, Casselman AL, Reppert SM (2003) Illuminating the circadian clock in monarch butterfly migration. Science 300:1303–1305. [DOI] [PubMed] [Google Scholar]
- 24.Guerra PA, Reppert SM (2013) Coldness triggers northward flight in remigrant monarch butterflies. Curr Biol 23:419–423. [DOI] [PubMed] [Google Scholar]
- 25.Merlin C, Gegear RJ, Reppert SM (2009) Antennal circadian clocks coordinate sun compass orientation in migratory monarch butterflies. Science 325:1700–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu H, Gegear RJ, Casselman A, Kanginakudru S, Reppert SM (2009) Defining behavioral and molecular differences between summer and migratory monarch butterflies. BMC Biol 7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goehring L, Oberhauser KS (2002) Effects of photoperiod, temperature, and host plant age on induction of reproductive diapause and development time in Danaus plexippus. Ecol Entomol 27:674–685. [Google Scholar]
- 28.Altizer S, Davis AK (2010) Populations of Monarch butterflies with different migratory behaviors show divergence in wing morphology. Evolution 64:1018–1028. [DOI] [PubMed] [Google Scholar]
- 29.Zhan S, et al. (2014) The genetics of monarch butterfly migration and warning colouration. Nature 514:317–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang H, Peng J, Wang P, Risch NJ (2005) Estimation of individual admixture: Analytical and study design considerations. Genet Epidemiol 28:289–301. [DOI] [PubMed] [Google Scholar]
- 31.Pickrell JK, Pritchard JK (2012) Inference of population splits and mixtures from genome-wide allele frequency data. PLoS Genet 8:e1002967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maeckle M. (2018) Five monarch butterflies tagged and released at San Antonio Festival made it to Mexico. Texas Butterfly Ranch. Available at https://texasbutterflyranch.com/2018/04/25/five-monarch-butterflies-tagged-and-released-at-san-antonio-festival-made-it-to-mexico/. Accessed December 6, 2018.
- 33.Freedman MG, et al. (2018) Non-migratory monarch butterflies, Danaus plexippus (L.), retain developmental plasticity and a navigational mechanism associated with migration. Biol J Linn Soc Lond 123:265–278. [Google Scholar]
- 34.Zhan S, Merlin C, Boore JL, Reppert SM (2011) The monarch butterfly genome yields insights into long-distance migration. Cell 147:1171–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKenna A, et al. (2010) The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20:1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lefort V, Desper R, Gascuel O (2015) FastME 2.0: A comprehensive, accurate, and fast distance-based phylogeny inference program. Mol Biol Evol 32:2798–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Whole-genome sequences are available at the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/bioproject) under BioProject accession number PRJNA509269. All other data are available in Dataset S1.