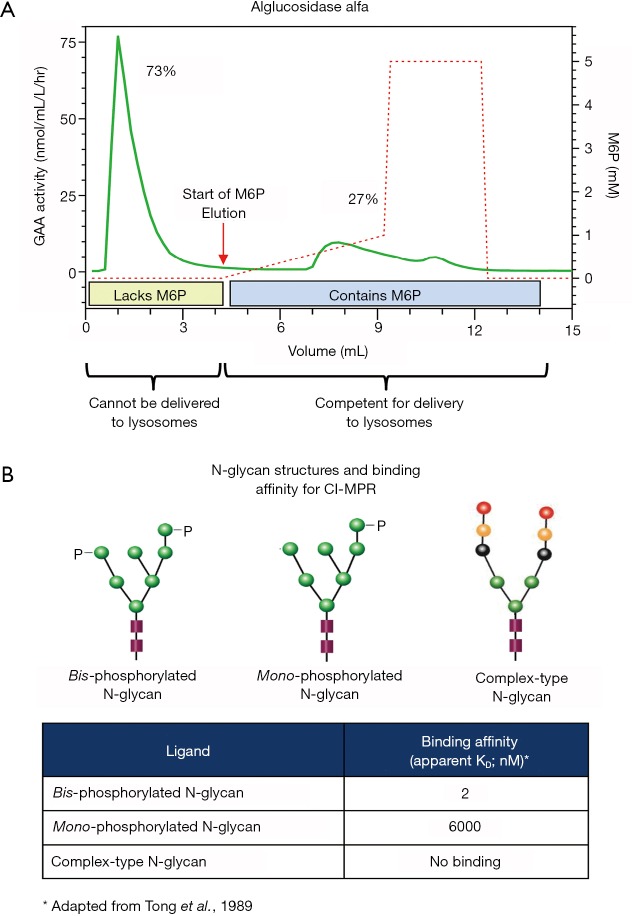
Figure 2.
(A) Alglucosidase alfa was loaded onto a CI-MPR column to assess the relative proportion of the enzyme mixture that contains M6P. rhGAA lacking M6P cannot bind to the CI-MPR and, therefore, flowed through the column, while rhGAA containing M6P binds to the CI-MPR and was retained on the column. The bound rhGAA was then eluted from the column using a linear gradient of increasing free M6P. The beginning of the linear gradient is indicated by the black arrows. Both fractions of rhGAA (unbound and bound/eluted) were collected and assayed for GAA enzyme activity using the fluorogenic substrate 4-methylumbeliferyl-α-glucopyranoside (4-MU-raGlc) to determine the relative percentage of rhGAA in each peak. (B) Representative N-linked oligosaccharide structures and their respective binding affinities for the CI-MPR. The binding affinities for radiolabeled bis- and mono-phosphorylated high mannose oligosaccharides and complex-type oligosaccharides were experimentally determined by equilibrium dialysis as reported by Tong et al. [1989]. Bis-phosphorylated high mannose oligosaccharides have very high affinity while mono-phosphorylated high mannose oligosaccharides have moderate affinity for the CI-MPR. Complex-type oligosaccharides and non-phosphorylated high mannose oligosaccharides (not shown) do not bind CI-MPR. rhGAA has 7 potential N-glycosylation sites (Park et al., 2018) and different enzyme preparations have varying amounts of bis- and mono-phosphorylated high mannose, non-phosphorylated high mannose and complex-type oligosaccharides. rhGAA, recombinant human acid alpha-glucosidase.