Abstract
Gene therapy for Pompe disease has advanced to early phase clinical trials, based upon proof-of-concept data indicating that gene therapy could surpass the benefits of the current standard of care, enzyme replacement therapy (ERT). ERT requires frequent infusions of large quantities of recombinant human acid α-glucosidase (GAA), whereas gene therapy involves a single infusion of a vector that stably transduces tissues to continuously produce GAA. Liver-specific expression of GAA with an adeno-associated virus (AAV) vector established stable GAA secretion from the liver accompanied by receptor-mediated uptake of GAA, which corrected the deficiency of GAA and cleared the majority of accumulated glycogen in the heart and skeletal muscle. Liver depot gene therapy was equivalent to ERT at a dose of the AAV vector that could be administered in an early phase clinical trial. Furthermore, high-level expression of GAA has decreased glycogen stored in the brain. A unique advantage of liver-specific expression stems from the induction of immune tolerance to GAA following AAV vector administration, thereby suppressing anti-GAA antibodies that otherwise interfere with efficacy. A Phase I clinical trial of AAV vector-mediated liver depot gene therapy has been initiated based upon promising preclinical data (NCT03533673). Overall, gene therapy promises to address limits of currently available ERT, if clinical translation currently underway is successful.
Keywords: Pompe disease, glycogen storage disease, adeno-associated virus (AAV) vector, gene therapy
Current therapy for Pompe disease
With the advent of enzyme replacement therapy (ERT; alglucosidase alfa, Myozyme, Lumizyme), patients with Pompe disease are surviving longer with improved clinical outcomes and better quality of life. Importantly, ERT has resolved and/or reduced many complications associated with Pompe, such as cardiomyopathy (particularly in the classic infantile-onset form of the disease), respiratory insufficiency requiring ventilator support, and general muscle weakness/motor disability status (1,2). Patients with infantile Pompe disease (IPD) are now surviving well into adolescence, and with advancements such as newborn screening (NBS), late-onset Pompe disease (LOPD) patients are being identified and monitored for symptom presentation starting at a very young age. As such, a new phenotype is emerging across the disease spectrum. Although many great strides have been made throughout the field, more than a decade of experience in the treatment and management of Pompe patients has also demonstrated that many unmet needs remain.
A better understanding of the pathophysiology of multisystem involvement in patients with Pompe has also provided valuable insight into the complications that still remain despite treatment with ERT. Postmortem identification of glycogen accumulation in many of the involved tissues can be correlated with persistent clinical symptoms and manifestations identified in patients living with Pompe disease. In IPD, cardiac arrhythmias have been correlated with vacuolation of the bundle of His as well as in the sinoatrial and atrioventricular nodes (3,4). Glycogen accumulation in smooth muscle of muscular arteries, esophagus, GI tract, and urinary bladder may be associated with reports of basilar artery aneurysm, dilatation of the ascending aorta, dysphagia, urinary incontinence, and gastrointestinal involvement (3,5). Additionally, refractory errors such as strabismus, myopia, and astigmatism may be related to glycogen accumulation found in ocular structures such as the extraocular muscles, lens epithelial cells, and retinal ganglion cells (3,6). While the extent of cognitive and/or intellectual deficits in IPD are not yet well-defined, glycogen accumulation has also been found in tissues of the nervous system such as anterior horn cells, glial cells, and astrocytes of white matter, which may be associated with white matter changes and lesions observed in MRI studies of IPD survivors (3,5,7).
There are relatively fewer autopsy reports available in LOPD patients treated with ERT. In one report, postmortem histological examination revealed severe glycogen accumulation in striated muscle, as well as severe fibrosis and vacuolar degeneration in intercostal, paravertebral, upper esophagus, and diaphragm muscles, associated with respiratory insufficiency that was identified as the primary cause of death alongside multi-system organ failure (8). No glycogen accumulation, however, was found in cardiac muscle, central nervous system, kidney, liver, spleen, or ileum. In another case report, postmortem examination similarly revealed extensive vacuolar myopathy in skeletal muscle (9). Other pathologic findings in this patient included glycogen-filled vacuoles within vascular smooth muscle, vacuolar change in the alimentary tract and urinary bladder, a moderate amount of glycogen accumulation present in Schwann cells of both central and peripheral nerves, and prominent lipofuscin pigment present within cardiomyocytes.
Taken together, along with need for very high dose of ERT and the frequency of the infusions and the clinical and pathologic findings in patients with IPD and LOPD demonstrate the need for next-generation targeted therapies that are able to specifically resolve these persistent manifestations, as well as improved, standardized biomarkers and clinical endpoints to assess the efficacy of treatment in patients with varying degrees of involvement.
Introduction to liver depot gene therapy
The marketing approval of LUXTERNA™ by the United States in 2018 (10) constitutes the advent of gene therapy for the treatment of genetic disease. Other gene therapies are in late stage clinical trials with the potential for Food and Drug Agency (FDA) approval in the near future (11). Thus, the groundwork has been laid for the development of gene therapy in other genetic diseases, including Pompe disease, where preclinical studies predict safety and clinical efficacy (12).
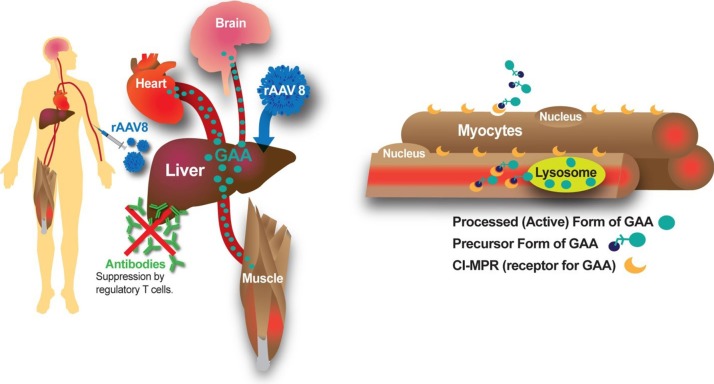
One well-validated approach to gene therapy in Pompe disease can be termed liver depot gene therapy (Table 1). The first report of liver depot gene therapy from Amalfitano and colleagues demonstrated the underlying principle of high-level liver expression of α-glucosidase (GAA), from a modified adenovirus vector in that study, accompanied by secretion to the blood stream and receptor-mediated uptake in the target tissues consisting of the heart and skeletal muscle (13). That study confirmed the presence of high molecular weight precursor acid GAA in the blood, which was taken up and processed to mature GAA in the heart and skeletal muscle where accumulated lysosomal glycogen was cleared very effectively. Although the GAA expression for liver proved to be transient, adenovirus vector-mediated GAA expression from the liver depot achieved high-level biochemical correction throughout the heart and skeletal muscle (23). The liver depot concept was further confirmed by other studies, which also revealed the complication of anti-GAA (IgG) antibodies that interfered with the biochemical correction of muscle by circulating GAA (23). Furthermore, anti-GAA antibodies could be reduced by including a liver-specific regulatory cassette to drive GAA expression (14). The strategy of gene therapy with adenovirus vectors has been further developed by constructing fully-deleted adenovirus vectors that were less likely to provoke high titer anti-GAA antibody or cytotoxic T lymphocyte (CTL) responses, and achieved longer term GAA expression (14). The fully deleted adenovirus was evaluated in a toxicology study in baboons and achieved GAA expression in plasma for over 6 months without significant toxicity (15). Overall, these studies confirmed that liver transduction to create a depot could achieve wide-spread biochemical correction including the heart and skeletal muscle through cation-independent mannose-6-phoshate receptor (CI-MPR) mediated uptake of precursor GAA and trafficking to the lysosomes, where GAA was processed and cleared accumulated glycogen stores (Figure 1).
Table 1. Liver depot therapy in Pompe disease.
| Vector | Regulatory cassette | GAA cDNA | Model | Efficacy | Antibody response | CTL |
|---|---|---|---|---|---|---|
| Modified adenovirus (13) | Cytomegalovirus promoter | Wildtype human | GAA-KO adult mice | Yes | Not reported | Not reported |
| Fully deleted adenovirus (14,15) | Phosphoenolpyruvate, carboxykinase promoter, apolipoprotein E enhancer | Wildtype human or baboon | GAA-KO adult mice and baboons | Yes (waning by 180–300 days) | Yes | No |
| AAV2/8 (16) | CB (chicken β-actin promoter/cytomegalovirus enhancer) | Wildtype human | GAA-KO adult mice | No | Present | Yes |
| AAV2/8 hGAA (17) | Duck hepatitis B virus (nonspecific) | Wildtype human | GAA-KO neonatal mice | Yes, if seronegative | Sporadic | No |
| AAV2/8 (16,18,19) | LSP (liver-specific promoter, a thyroid hormone-binding globulin promoter sequence downstream from 2 copies of a α1-microglobulin/bikunin enhancer) | Wildtype human | GAA-KO adult mice | Yes (immunomodulatory gene therapy) | Absent | No |
| AAV2/8 (20) | LSP | Secreted human | GAA-KO adult mice | Yes | Absent | No |
| AAV2/8 (21) | DC190 (liver-specific promoter) | Wildtype human | GAA-KO adult mice, including old mice | Yes (decreased in old mice) | Absent | No |
| AAV2/8 (22) | Human α1-antitrypsin promoter, apolipoprotein E enhancer | Secreted human | GAA-KO adult mice, and non-human primates (Macaca fascicularis) | Yes | Absent | Not reported |
GAA, α-glucosidase; CTL, cytotoxic T lymphocyte; GAA-KO, GAA-knockout; AAV, adeno-associated virus.
Figure 1.
Liver depot gene therapy for Pompe disease. Liver depot gene therapy suppresses immune responses and corrects GAA deficiency in the muscle and brain through receptor-mediated uptake of secreted GAA precursor from the blood. The 110 kD precursor GAA is trafficked to the lysosomes with the CI-MPR, where it matures to the 76 kD activated GAA. Liver-specific expression activates GAA-specific regulatory T cells that suppress anti-GAA antibodies. GAA, α-glucosidase; CI-MPR, cation-independent mannose-6-phoshate receptor.
More recently adeno-associated virus (AAV) vectors have been widely adopted for liver transduction to produce secreted proteins including coagulation factors and lysosomal enzymes (24-26), including in Pompe disease (16). The concept of AAV vector-mediated liver-specific transgene expression to suppress antibody responses against therapeutic proteins was developed first in hemophilia models (27,28) and later in Fabry (26) and Pompe disease (16,29). Immune challenges to ERT and other gene therapy approaches using a liver specific promoter confirmed that immune tolerance to GAA could be induced by liver-specific expression (16,21,29). Furthermore, low-dose AAV vector administration could induce immune tolerance to GAA that enhanced the efficacy from simultaneous ERT (29,30), which both improved the biochemical correction from ERT and prevented hypersensitivity reactions by suppressing anti-GAA antibody formation. The latter effect has been termed immunomodulatory gene therapy (31), and the underlying mechanism is the activation of regulatory T cells that suppress antibody responses against GAA (18) (Figure 1).
Advantages of liver depot gene therapy for Pompe disease
The potential for liver depot gene therapy to surpass ERT was clear from early proof-of-concept studies that demonstrated correction of GAA deficiency and clearance of lysosomal glycogen in skeletal muscle (13,16,23). Importantly, liver depot gene therapy can correct type II myofiber muscles that resist correction from ERT (32,33). Later studies suggested the feasibility of clearing sequestered glycogen from the central nervous system following high-level hepatic GAA production (12,22). The latter effect can be attributed to CI-MPR mediated transfer of a lysosomal enzyme such as GAA across the blood-brain barrier (34). The minimum effective dose for an AAV vector to achieve biochemical correction from a liver depot has been reported as 8E+10 vector genomes (vg) per kg body weight for the heart and diaphragm, and 8E+11 vg/kg for skeletal muscle (19), although these minimum effective dosages will depend upon vector design. One clear advantage of gene therapy over ERT stems from the continuous, low-level exposure of skeletal muscle to GAA from the liver depot, in contrast to periodic, high-level exposure from ERT (19). Additionally, liver depot gene therapy can prevent or suppress the complication of anti-GAA antibody formation following ERT that interferes with biochemical correction (19,29).
Liver depot therapy has advantages due to the very high tropism of AAV vectors for the liver, which reduces the dose requirements for gene therapy in Pompe disease, in comparison with muscle-targeted gene therapy. Muscle-targeted gene therapy has been attempted by incorporating a highly active muscle-specific regulatory cassette (MHCK7) in a vector encoding GAA (35). Although this vector expressed supraphysiologic GAA activity in skeletal muscle and substantially cleared lysosomal glycogen, dose requirements were high. The dose needed to substantially clear lysosomal glycogen from skeletal muscle was estimated at 2E+13 vg/kg for a recombinant (r) AAV9 vector containing MHCK7 (at least >4E+12 vg/kg) in adult mice with Pompe disease (35), which was much higher than the highly effective dose for liver depot gene therapy that has been estimated to be 2E+12 vg/kg in the same strain of mice (19). A similar effect was achieved by a rAAV9 vector containing a desmin promoter at a higher dose (approximately 4E+13 vg/kg), confirming the high dose requirements for direct muscle transduction in Pompe disease (36).
Immune responses against GAA can be suppressed by liver depot therapy, whereas non-specific GAA expression with systemic gene therapy provokes neutralizing immune responses in adult GAA knockout (KO) mice (16,18). The disadvantages of non-specific GAA expression were clearly demonstrated in a direct comparison of rAAV8 vectors containing a liver-specific promoter (LSP) or the CMV enhancer-chicken β-actin (CB) promoter (16). Whereas both vectors expressed GAA that was detected in plasma in the days following vector administration, GAA expressed from the CB-containing vector disappeared at day 14 when anti-GAA IgG antibodies appeared. The CB-containing vector also provoked CTL responses characterized by CD8+ lymphocytes in skeletal muscle, and a positive ELISPOT response against GAA at day 14. Intriguingly, immune responses against systemic GAA expression can be suppressed by simultaneous administration of an AAV vector expressing GAA specifically in the liver to induce immune tolerance to GAA (18,37).
Methods to enhance the benefits of liver depot gene therapy in Pompe disease
Efforts to enhance the efficacy from liver depot gene therapy have included increased GAA secretion from the liver and increased CI-MPR expression in skeletal muscle. These methods have been successful at improving the biochemical correction of skeletal muscle from low dosages of the AAV vector encoding GAA. Alternatively, increased vector dosing might increase efficacy from liver depot therapy.
The initial study of enhancing secretion of GAA used a strategy that modified the signal peptide of GAA to that of a highly secreted protein to produce a chimeric, secreted GAA, replicating earlier work that illustrated feasibility for that technique (20,38). This strategy increased the secretion of chimeric GAA from transfected HEK 293 cells by up to 26-fold. Receptor-mediated uptake of secreted, chimeric GAA corrected cultured Pompe disease patient cells. High-level hGAA was sustained in the plasma of mice with Pompe disease for 24 weeks following administration of a rAAV8 vector encoding chimeric GAA; furthermore, GAA activity was increased and glycogen content was significantly reduced in striated muscle and in the brain. Administration of only 4E+11 vg/kg vector particles increased GAA activity in the heart and diaphragm for >18 weeks, whereas 1.2E+12 vg/kg vector particles increased GAA activity and reduced glycogen content in the heart, diaphragm, and quadriceps. These data confirmed the feasibility of modifying GAA to drive secretion from transduced hepatocytes, thereby increasing the availability of GAA for the cross-correction of skeletal muscle.
A more recent study of chimeric GAA confirmed the strategy of modifying the signal peptide, and that of deletion of at least eight amino acids in the N terminus of the propeptide region of the enzyme, to enhance the secretion from the liver (22). The GAA cDNA was codon-optimized to increase GAA expression; however, the investigators unexpectedly detected anti-GAA antibodies in response to liver expression of the codon-optimized GAA containing the native signal peptide at vector dosages of 5E+11 vg/kg and 2E+12 vg/kg. The combined modifications of altering the signal peptide and codon-optimization prevented anti-GAA formation at those dosages. Overall, liver production of the modified GAA reduced accumulated glycogen in skeletal muscle and the brain, decreased cardiac hypertrophy, and improved muscle and respiratory function in mice with Pompe disease.
Another strategy to enhance the efficacy of liver depot therapy in Pompe disease consists of enhancing the expression of CI-MPR in skeletal muscle to increase the uptake of GAA from the blood stream (33,39). CI-MPR was increased by the administration of the long-acting, selective β2-agonist clenbuterol, repurposed from the treatment of asthma to provide an adjunctive therapy in Pompe disease. The efficacy of liver depot gene therapy was enhanced by the addition of clenbuterol, as demonstrated by increased Rotarod latency, in comparison with vector alone. Glycogen content was lower in skeletal muscles, including soleus (comprised of type I myofibers), extensor digitorum longus (comprised of type II myofibers) and tibialis anterior (type II myofibers) following combination therapy, in comparison with vector treatment alone. Glycogen was decreased but remained elevated in the muscles following clenbuterol alone, indicating an adjunctive effect with gene therapy. Elderly mice treated with combination therapy demonstrated increased wirehang latency, in comparison with vector or clenbuterol treatment alone, further demonstrating a synergistic effect of the combination therapy (33).
Clinical translation of liver depot gene therapy for Pompe disease
Currently two major obstacles must be overcome to demonstrate the feasibility of using gene therapy to treat Pompe disease, and generally for gene therapy to become broadly applicable in the treatment of all genetic diseases. Pre-existing anti-AAV antibodies and the induction of a de novo antibody response remain an obstacle for initiating gene therapy, and for re-administration of AAV vectors that might be needed eventually to maintain efficacy (40). Re-administration will be needed at least for young patients, who will eventually need to be re-treated due to cell division during growth and the gradual loss of episomal AAV genomes. Given that these are critical to the entire field, we have focused upon clinical development of liver depot gene therapy for Pompe disease, recently initiating a Phase I clinical trial (NCT03533673).
Dose requirements represent a critical factor in establishing clinical feasibility. Several factors determine the dose requirements for correction of GAA deficiency in Pompe disease. Patient characteristics include the degree of GAA deficiency, the age at which the patient receives treatment, the need for correction of the large mass of skeletal muscle, the stage of disease progression, and the immune response to GAA. Vector characteristics include the choice of AAV serotype, the promoter that determines the tissue from which GAA is expressed, and any modifications to the GAA coding sequence (such as the signal peptide). We have developed liver depot gene therapy with a rAAV8 vector containing an LSP to drive wildtype GAA expression. Rather than frequent infusions of a recombinant protein, as in ERT, gene therapy with a rAAV8 vector will be performed once with long-lasting effects. For example, rAAV8 vector-mediated gene therapy in adult males with hemophilia B has had sustained, dose-related benefits for greater than 3 years (41). The expectation is that the treatment of very young patients would have lower, possibly time-limited benefits due to the loss of episomal AAV vector genomes during growth (42).
In the initial clinical application of immunomodulatory gene therapy, liver-specific expression of the therapeutic protein will prevent neutralizing antibody responses against the therapeutic protein even at low vector dosages (19). This strategy will induce specific immune tolerance to GAA in Pompe disease with a low dose of an rAAV8 vector containing a LSP that expressed GAA only in liver and induced immune tolerance to GAA, AAV2/8-LSPhGAA (29,30), thereby providing safe immune tolerance induction without the risk for immune suppression or other toxic effects of current drug regimens (43). Additionally, a low dose of AAV2/8-LSPhGAA improved the efficacy of concurrent ERT. Preclinical experiments optimized the dosing of AAV2/8-LSPhGAA in adult immunocompetent mice with Pompe disease in which biochemical correction of skeletal muscle was achieved most effectively with a moderate dose requirement (2×1012 vg/kg) (19). This dose was 10-fold higher than a minimum effective dose of 2×1011 vg/kg that partially corrected GAA deficiency in skeletal muscle and induced immune tolerance to GAA ERT (19). This preclinical data justified a starting dose of 1.6×1012 vg/kg for our Phase I clinical trial, given that the established threshold for safety was 10-fold higher (12). Further work is needed in establishing the right dose, and also the ability to treat infantile Pompe disease.
Conclusions
The future for liver depot gene therapy looks promising, including the outlook for clinical development in Pompe disease. Currently available data predicts favorable outcomes from early stage clinical trials, given the advantages of liver depot gene therapy that promise to address the limitations of ERT. Strategies are available to enhance the effect of liver depot gene therapy. The dose requirements of liver depot gene therapy in Pompe disease are consistent with other successful gene therapies that are further along in clinical development. Remaining hurdles for the field include the presence of AAV antibodies in the population that exclude patients from clinical trials and prevent re-administration, as well as the need for enhanced methods to treat young patients. Overall, currently available data indicate that clinical trials evaluating the safety and efficacy of liver depot gene therapy are warranted in patients with Pompe disease.
Acknowledgments
We wish to acknowledge Ms. Cindy Li for assistance with the literature search.
Funding: This work was supported by NIH Grant R01AR065873 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Footnotes
Conflicts of Interest: DD Koeberl, PS Kishnani, and Duke University might benefit financially, if the experimental treatments discussed here prove effective and are successful commercially. DD Koeberl, PS Kishnani, and Duke University have equity in Actus Therapeutics, which is developing gene therapy for Pompe disease. Additionally, PS Kishnani has received research/grant support from Sanofi Genzyme, Valerion Therapeutics, and Amicus Therapeutics; has received consulting fees and honoraria from Sanofi Genzyme, Amicus Therapeutics, Vertex Pharmaceuticals and Asklepios Biopharmaceutical, Inc. (AskBio); is a member of the Pompe and Gaucher Disease Registry Advisory Board for Sanofi Genzyme, Amicus Therapeutics, and Baebies.
References
- 1.Hahn SH, Kronn D, Leslie ND, et al. Efficacy, safety profile, and immunogenicity of alglucosidase alfa produced at the 4,000-liter scale in US children and adolescents with Pompe disease: ADVANCE, a phase IV, open-label, prospective study. Genet Med 2018;20:1284-94. 10.1038/gim.2018.2 [DOI] [PubMed] [Google Scholar]
- 2.Thurberg BL, Lynch Maloney C, Vaccaro C, et al. Characterization of pre- and post-treatment pathology after enzyme replacement therapy for Pompe disease. Lab Invest 2006;86:1208-20. 10.1038/labinvest.3700484 [DOI] [PubMed] [Google Scholar]
- 3.Pena LD, Proia AD, Kishnani PS. Postmortem Findings and Clinical Correlates in Individuals with Infantile-Onset Pompe Disease. JIMD Rep 2015;23:45-54. 10.1007/8904_2015_426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakamura Y, Tanimura A, Yasuoka C, et al. An autopsy case of type II glycogenosis. Kurume Med J 1979;26:349-54. 10.2739/kurumemedj.26.349 [DOI] [PubMed] [Google Scholar]
- 5.Sakurai I, Tosaka A, Mori Y, et al. Glycogenosis type II (Pompe). The fourth autopsy case in Japan. Acta Pathol Jpn 1974;24:829-46. [DOI] [PubMed] [Google Scholar]
- 6.Prakalapakorn SG, Proia AD, Yanovitch TL, et al. Ocular and histologic findings in a series of children with infantile pompe disease treated with enzyme replacement therapy. J Pediatr Ophthalmol Strabismus 2014;51:355-62. 10.3928/01913913-20140813-01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teng YT, Su WJ, Hou JW, et al. Infantile-onset glycogen storage disease type II (Pompe disease): report of a case with genetic diagnosis and pathological findings. Chang Gung Med J 2004;27:379-84. [PubMed] [Google Scholar]
- 8.Kobayashi H, Shimada Y, Ikegami M, et al. Prognostic factors for the late onset Pompe disease with enzyme replacement therapy: from our experience of 4 cases including an autopsy case. Mol Genet Metab 2010;100:14-9. 10.1016/j.ymgme.2010.01.015 [DOI] [PubMed] [Google Scholar]
- 9.Hobson-Webb LD, Proia AD, Thurberg BL, et al. Autopsy findings in late-onset Pompe disease: a case report and systematic review of the literature. Mol Genet Metab 2012;106:462-9. 10.1016/j.ymgme.2012.05.007 [DOI] [PubMed] [Google Scholar]
- 10.Rodrigues GA, Shalaev E, Karami TK, et al. Pharmaceutical Development of AAV-Based Gene Therapy Products for the Eye. Pharm Res 2018;36:29. 10.1007/s11095-018-2554-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendell JR, Al-Zaidy S, Shell R, et al. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N Engl J Med 2017;377:1713-22. 10.1056/NEJMoa1706198 [DOI] [PubMed] [Google Scholar]
- 12.Wang G, Young SP, Bali D, et al. Assessment of toxicity and biodistribution of recombinant AAV8 vector-mediated immunomodulatory gene therapy in mice with Pompe disease. Mol Ther Methods Clin Dev 2014;1:14018. 10.1038/mtm.2014.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amalfitano A, McVie-Wylie AJ, Hu H, et al. Systemic correction of the muscle disorder glycogen storage disease type II after hepatic targeting of a modified adenovirus vector encoding human acid-alpha-glucosidase. Proc Natl Acad Sci U S A 1999;96:8861-6. 10.1073/pnas.96.16.8861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiang A, Hartman ZC, Liao SX, et al. Fully deleted adenovirus persistently expressing GAA accomplishes long-term skeletal muscle glycogen correction in tolerant and nontolerant GSD-II mice. Mol Ther 2006;13:127-34. 10.1016/j.ymthe.2005.08.006 [DOI] [PubMed] [Google Scholar]
- 15.Rastall DP, Seregin SS, Aldhamen YA, et al. Long-term, high-level hepatic secretion of acid alpha-glucosidase for Pompe disease achieved in non-human primates using helper-dependent adenovirus. Gene Ther 2016;23:743-52. 10.1038/gt.2016.53 [DOI] [PubMed] [Google Scholar]
- 16.Franco LM, Sun B, Yang X, et al. Evasion of immune responses to introduced human acid alpha-glucosidase by liver-restricted expression in glycogen storage disease type II. Mol Ther 2005;12:876-84. 10.1016/j.ymthe.2005.04.024 [DOI] [PubMed] [Google Scholar]
- 17.Cresawn KO, Fraites TJ, Wasserfall C, et al. Impact of humoral immune response on distribution and efficacy of recombinant adeno-associated virus-derived acid alpha-glucosidase in a model of glycogen storage disease type II. Hum Gene Ther 2005;16:68-80. 10.1089/hum.2005.16.68 [DOI] [PubMed] [Google Scholar]
- 18.Zhang P, Sun B, Osada T, et al. Immunodominant liver-specific expression suppresses transgene-directed immune responses in murine pompe disease. Hum Gene Ther 2012;23:460-72. 10.1089/hum.2011.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han SO, Ronzitti G, Arnson B, et al. Low-Dose Liver-Targeted Gene Therapy for Pompe Disease Enhances Therapeutic Efficacy of ERT via Immune Tolerance Induction. Mol Ther Methods Clin Dev 2017;4:126-36. 10.1016/j.omtm.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun B, Zhang H, Benjamin DK, Jr, et al. Enhanced efficacy of an AAV vector encoding chimeric, highly secreted acid alpha-glucosidase in glycogen storage disease type II. Mol Ther 2006;14:822-30. 10.1016/j.ymthe.2006.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ziegler RJ, Bercury SD, Fidler J, et al. Ability of adeno-associated virus serotype 8-mediated hepatic expression of acid alpha-glucosidase to correct the biochemical and motor function deficits of presymptomatic and symptomatic Pompe mice. Hum Gene Ther 2008;19:609-21. 10.1089/hum.2008.010 [DOI] [PubMed] [Google Scholar]
- 22.Puzzo F, Colella P, Biferi MG, et al. Rescue of Pompe disease in mice by AAV-mediated liver delivery of secretable acid alpha-glucosidase. Sci Transl Med 2017;9(418). 10.1126/scitranslmed.aam6375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding E, Hu H, Hodges BL, et al. Efficacy of gene therapy for a prototypical lysosomal storage disease (GSD-II) is critically dependent on vector dose, transgene promoter, and the tissues targeted for vector transduction. Mol.Ther. 2002;5:436-46. 10.1006/mthe.2002.0563 [DOI] [PubMed] [Google Scholar]
- 24.Snyder RO, Miao C, Meuse L, et al. Correction of hemophilia B in canine and murine models using recombinant adeno-associated viral vectors. Nat Med 1999;5:64-70. 10.1038/4751 [DOI] [PubMed] [Google Scholar]
- 25.Burton M, Nakai H, Colosi P, et al. Coexpression of factor VIII heavy and light chain adeno-associated viral vectors produces biologically active protein. Proc Natl Acad Sci U S A 1999;96:12725-30. 10.1073/pnas.96.22.12725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ziegler RJ, Lonning SM, Armentano D, et al. AAV2 vector harboring a liver-restricted promoter facilitates sustained expression of therapeutic levels of alpha-galactosidase A and the induction of immune tolerance in Fabry mice. Mol Ther 2004;9:231-40. 10.1016/j.ymthe.2003.11.015 [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Nichols TC, Read MS, et al. Sustained expression of therapeutic level of factor IX in hemophilia B dogs by AAV-mediated gene therapy in liver. Mol Ther 2000;1:154-8. 10.1006/mthe.2000.0031 [DOI] [PubMed] [Google Scholar]
- 28.Harding TC, Koprivnikar KE, Tu GH, et al. Intravenous administration of an AAV-2 vector for the expression of factor IX in mice and a dog model of hemophilia B. Gene Ther 2004;11:204-13. 10.1038/sj.gt.3302142 [DOI] [PubMed] [Google Scholar]
- 29.Sun B, Bird A, Young SP, et al. Enhanced response to enzyme replacement therapy in pompe disease after the induction of immune tolerance. Am J Hum Genet 2007;81:1042-9. 10.1086/522236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun B, Kulis MD, Young SP, et al. Immunomodulatory gene therapy prevents antibody formation and lethal hypersensitivity reactions in murine pompe disease. Mol Ther 2010;18:353-60. 10.1038/mt.2009.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bond JE, Kishnani PS, Koeberl DD. Immunomodulatory, liver depot gene therapy for Pompe disease. Cell Immunol 2017. [Epub ahead of print]. 10.1016/j.cellimm.2017.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raben N, Fukuda T, Gilbert AL, et al. Replacing acid alpha-glucosidase in Pompe disease: Recombinant and transgenic enzymes are equipotent, but neither completely clears glycogen from type II muscle fibers. Mol Ther 2005;11:48-56. 10.1016/j.ymthe.2004.09.017 [DOI] [PubMed] [Google Scholar]
- 33.Li S, Sun B, Nilsson MI, et al. Adjunctive beta2-agonists reverse neuromuscular involvement in murine Pompe disease. FASEB J 2013;27:34-44. 10.1096/fj.12-207472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urayama A, Grubb JH, Sly WS, et al. Developmentally regulated mannose 6-phosphate receptor-mediated transport of a lysosomal enzyme across the blood-brain barrier. Proc Natl Acad Sci U S A 2004;101:12658-63. 10.1073/pnas.0405042101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun B, Young SP, Li P, et al. Correction of multiple striated muscles in murine Pompe disease through adeno-associated virus-mediated gene therapy. Mol.Ther. 2008;16:1366-71. 10.1038/mt.2008.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keeler AM, Zieger M, Todeasa SH, et al. Systemic Delivery of AAVB1-GAA Clears Glycogen and Prolongs Survival in a Mouse Model of Pompe Disease. Hum Gene Ther 2019;30:57-68. 10.1089/hum.2018.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doerfler PA, Todd AG, Clement N, et al. Copackaged AAV9 Vectors Promote Simultaneous Immune Tolerance and Phenotypic Correction of Pompe Disease. Hum Gene Ther 2016;27:43-59. 10.1089/hum.2015.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barash S, Wang W, Shi Y. Human secretory signal peptide description by hidden Markov model and generation of a strong artificial signal peptide for secreted protein expression. Biochem Biophys Res Commun 2002;294:835-42. 10.1016/S0006-291X(02)00566-1 [DOI] [PubMed] [Google Scholar]
- 39.Koeberl DD, Luo X, Sun B, et al. Enhanced efficacy of enzyme replacement therapy in Pompe disease through mannose-6-phosphate receptor expression in skeletal muscle. Mol Genet Metab 2011;103:107-12. 10.1016/j.ymgme.2011.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halbert CL, Standaert TA, Wilson CB, et al. Successful Readministration of Adeno-associated virus vectors to the mouse lung requires transient immunosuppression during the initial exposure. J Virol 1998;72:9795-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nathwani AC, Reiss UM, Tuddenham EG, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med 2014;371:1994-2004. 10.1056/NEJMoa1407309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cunningham SC, Dane AP, Spinoulas A, et al. Gene delivery to the juvenile mouse liver using AAV2/8 vectors. Mol Ther 2008;16:1081-8. 10.1038/mt.2008.72 [DOI] [PubMed] [Google Scholar]
- 43.Messinger YH, Mendelsohn NJ, Rhead W, et al. Successful immune tolerance induction to enzyme replacement therapy in CRIM-negative infantile Pompe disease. Genet Med 2012;14:135-42. 10.1038/gim.2011.4 [DOI] [PMC free article] [PubMed] [Google Scholar]