Abstract
Botanical dietary supplements for women’s health are increasingly popular. Older women tend to take botanical supplements such as hops as natural alternatives to traditional hormone therapy to relieve menopausal symptoms. Especially extracts from spent hops, the plant material remaining after beer brewing, are enriched in bioactive prenylated flavonoids that correlate with the health benefits of the plant. The chalcone xanthohumol (XH) is the major prenylated flavonoid in spent hops. Other less abundant but important bioactive prenylated flavonoids are isoxanthohumol (IX), 8-prenylnaringenin (8-PN), and 6-prenylnaringenin (6-PN). Pharmacokinetic studies revealed that these flavonoids are conjugated rapidly with glucuronic acid. XH also undergoes phase I metabolism in vivo to form IX, 8-PN, and 6-PN. Several hop constituents are responsible for distinct effects linked to multiple biological targets, including hormonal, metabolic, inflammatory, and epigenetic pathways. 8-PN is one of the most potent phytoestrogens and is responsible for hops’ estrogenic activities. Hops also inhibit aromatase activity, which is linked to 8-PN. The weak electrophile, XH, can activate the Keap1-Nrf2 pathway and turn on the synthesis of detoxification enzymes such as NAD(P)H-quinone oxidoreductase 1 and glutathione S-transferase. XH also alkylates IKK and NF-κB, resulting in anti-inflammatory activity. Antiobesity activities have been described for XH and XH-rich hop extracts likely through activation of AMP-activated protein kinase signaling pathways. Hop extracts modulate the estrogen chemical carcinogenesis pathway by enhancing P450 1A1 detoxification. The mechanism appears to involve activation of the aryl hydrocarbon receptor (AhR) by the AhR agonist, 6-PN, leading to degradation of the estrogen receptor. Finally, prenylated phenols from hops are known inhibitors of P450 1A1/2; P450 1B1; and P450 2C8, 2C9, and 2C19. Understanding the biological targets of hop dietary supplements and their phytoconstituents will ultimately lead to standardized botanical products with higher efficacy, safety, and chemopreventive properties.
Graphical Abstract
1. INTRODUCTION
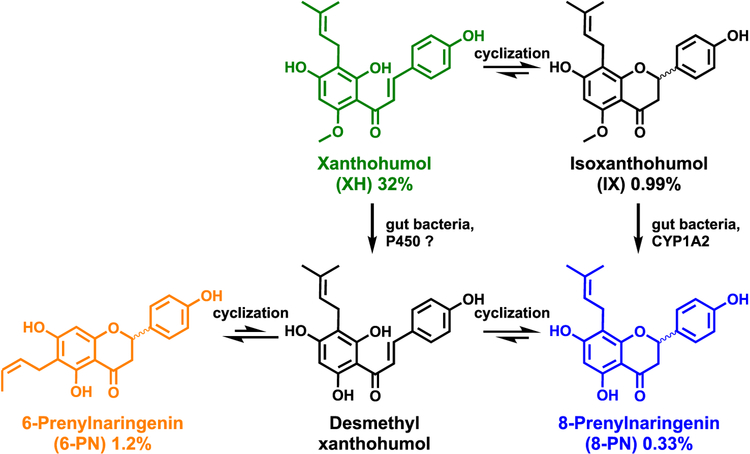
Hops, the strobili of Humulus lupulus L. (Cannabaceae), are added to beer during its preparation and are largely responsible for the taste.1–3 Hops are added in the form of dried plant material or as lipophilic extracts. Relatively hydrophilic hop extracts are also used in phytotherapy as one of the ingredients of herbal sedative/sleeping pills and as a natural alternative to traditional hormone replacement formulations to relieve menopausal symptoms.4–6 Hop extracts are generally obtained from the female hop cones, and the extracts used for the production of dietary supplements are particularly rich in prenylated phenols including chalcones and flavanones (“flavonoids;” Figure 1).1,7 This review will focus on preparations from spent hop cones, representing the material remaining after extraction of the much more lipophilic hop bitter acids, prenylated phloroglucinols, and the essential oils that are important for beer brewing.8 Xanthohumol (XH) is the most highly concentrated phenolic hop constituent. However, as all of the hop flavonoids are biochemically interconvertible (Figure 1), varying ratios of metabolites are observed depending on the extract preparation. XH has various anticarcinogenic properties, shown in vitro at key stages of the carcinogenic process including initiation, promotion, and progression phases.9,10 In addition to XH, many biological activities of spent hops have been connected to the other prenylated flavanones and chalcones (Figure 1) including 8-prenylnarigenin (8-PN), 6-prenylnarigenin (6-PN), and isoxanthohumol (IX).1,11
Figure 1.
Main bioactive compounds in the standardized spent hop extract. The concentrations of the compounds in the hop extract were determined as described in ref 8. P450 1A2 and the human gut bacterium Eubacterium limosum have been described to metabolize IX to 8-PN.25,26 While P450 mediated O-demethylation of XH to DMX is theoretically possible, it has not been analyzed in detail and might not be clinically relevant.20
This review evaluates the biological targets of spent hops and connects the designated major bioactive constituent to the respective bioactivity. After presenting the unique phytochemistry of spent hops, this review will summarize the current knowledge about pharmacokinetic data of spent hop extracts and their individual flavonoids/chalcones. Subsequently, the text covers the diverse biological targets starting with the action of 8-PN on the estrogen receptor (ER) and on aromatase, followed by 6-PN’s agonistic properties at the aryl hydrocarbon receptor (AhR), and XH’s chemopreventive as well as antiglycemic activities. Evidence for potential herb-drug interactions of hops are analyzed by looking at CYP P450 inhibition studies with hop extracts and prenylated phenols from hops. The final section provides a summary of methodology for optimizing spent hop extracts to contain enriched amounts of beneficial bioactive compounds and/or reduced levels of constituents with less desirable effects, collectively leading to hop extracts with enhanced efficacy and safety. It should be emphasized that hop extracts represent a metabolomic mixture that contains thousands of compounds, all of which are likely to have biological activities on their own or in combination with other compounds. This polypharmacological activity of hops is responsible for its rich biological history.3
2.a. Phytochemistry of Hops.
The term “hops” is diffuse as it may generally refer to one or more entire plant, or just to the strobili (female inflorescences) of Humulus lupulus L., or to any of a multitude of proprietary preparations used in brewing. Hop strobili are generally known in commerce as “hop cones,” whereas the proper pharmacognostic term is Strobili lupuli. Being distinct from the composition of the rest of the plant, the phytochemical constituents of Strobili lupuli have been studied extensively.12 Both chemical knowledge and product development have been mainly driven by the brewing industry, as hops have been considered an essential ingredient in beer for >500 years.13 The large economic footprint and inherent competition in the industry led to extensive research and rigorous optimization of the sensory properties of the hop bitter acids (prenylated phloroglucinol derivatives), from all aspects of hop production from agronomic factors such as breeding and farming practices through harvesting, drying, storage, extraction, formulation, and dosing. Initial development of hop extracts for brewing employed traditional solvent extraction. By the 1980s, hop extracts had become one of the driving forces in the development of the supercritical fluid extraction (SFE) industry. SFEs are regarded as a more stable, efficient, and consistent manner to impart hop bitterness into beer and are employed by most large breweries worldwide. Notably, employing hop SFEs in brewing produces large amounts of spent hops as a byproduct—which is well suited as a starting material for the biomedical research described herein.8
2.b. Dried Entire Hops vs Spent Hops.
Anecdotal observations of “side effects” associated with whole hops, such as estrogenic effects, have sparked further interest in the bioactivity and chemical diversity of the plant.13 Early investigators categorized hop constituents broadly into the volatile essential oil, “soft resins,” and “hard resins.”14 The essential oil and soft resins are responsible for most hop flavor in beer. The soft resins are primarily composed of the bitter acids along with waxes, fats, and entrained volatile terpenoids. The role of the hard resins in brewing and beer flavor has long been controversial. However, there is consensus that most of their constituents, and notably the key phytoestrogenic compound, 8-PN, as well as its precursors, remain in the plant material after CO2 SFE and, therefore, are not part of the prevalent beer brewing processes that rely on hop SFEs as ingredients. Accordingly, spent hops contain the entirety of the phytoestrogenic potential of this plant, which has primarily been attributed to 8-PN (Figure 1),15 a compound that remains the most potent phytoestrogen known to date. Other notable phytoconstituents of spent hops include the “protoestrogen” desmethylxanthohumol (DMX), which isomerizes to form 8-PN and/or the isomeric, 6-PN, IX, and its precursor XH (Figure 1). Collectively, these considerations make spent hops an ideal starting material for a botanical dietary supplement as it can be produced from the byproduct of a food manufacturing process, and because it has favorable health effects related to bioactive compounds that are much less important for or even unrelated to brewing beer.8
2.c. Phytochemistry of Spent Hops.
Among the many pharmacologically active components identified to date in spent hops, the prenylated chalcone, XH, is one of the most abundant and most widely studied phytoconstituents.5,7 This is due to both its abundance in hops and its attraction as a natural chemopreventive agent. An intramolecular Michael addition leads to isomerization and establishes both close biogenetical and chemical relationships between the hop chalcones (XH, DMX) and their corresponding flavanones (IX, 8-PN, 6-PN).16 To date, all evidence indicates that chalcones are the end of the actual biosynthetic pathways, whereas the flavanones arise through chemical isomerization of their natural chalcone precursors (Figure 1).
3. PK PROPERTIES OF HOP BIOACTIVE COMPOUNDS
Following oral administration, hop prenylated phenols are absorbed through the intestinal epithelium at a slow to moderate rate. Studies using the Caco-2 human intestinal epithelial model indicate that the flavonoid 8-PN crosses the intestinal epithelium at a moderate rate via passive diffusion,17 while the chalcone XH accumulates in intestinal epithelial cells and enters the general circulation more slowly.18 The Caco-2 cell monolayer model studies also indicated that glucuronidation as well as sulfation of these compounds can occur in the intestinal epithelium. Cell based studies using human liver microsomes and primary human hepatocytes have shown that phase II conjugation predominates over phase I metabolism and that glucuronidation is the primary route of conjugation.17,19
In clinical trials using orally administered extracts of spent hops or isolated hop prenylated phenols, conjugated metabolites were abundant in serum and urine, whereas only traces of unconjugated compounds or phase I metabolites (<10% of the administered dose) were observed.20,21 Similarly, after oral administration of a hop extract, no phase I metabolites but predominantly glucuronides of hop prenylated phenols and only traces of the aglycones were measured in human breast tissue.22
Urinary excretion of glucuronides and sulfates accounted for <2% of the administered dose of hop prenylated phenols in 24 h21 and <8% in 48 h.23 During the first 48 h after oral administration to humans, up to 24% of the administered doses of hop prenylated phenols were recovered in feces,23 indicating biliary secretion of conjugated metabolites followed by fecal elimination was the primary route of excretion. However, the slow rate of excretion suggested enterohepatic circulation.
The pharmacokinetics of hop bioactive compounds have been studied using isolated, single prenylated phenols as well as with extracts of spent hops containing mixtures.20,21,23 Similar pharmacokinetics results have been obtained for studies of mixtures as well as for investigations of individual hop compounds, indicating no evidence of PK interactions between hop constituents. The maximum serum concentrations of bioactive hop compounds were dose dependent and occurred 1 to 7 h after oral administration.20,21 Secondary peaks in serum concentration were observed 4 to 5 h later, indicating enterohepatic circulation. Slow absorption and enterohepatic circulation of these bioactive hop compounds resulted in halflives exceeding 20 h.20,21
Quantitative analysis of multiple bioactive hop compounds during pharmacokinetic studies using a standardized extract of spent hops in women21 indicated some cyclization of XH occurred to form IX as well as some demethylation of IX to form 8-PN. Human pharmacokinetics studies with pure XH confirmed in vivo formation of IX and trace amounts of the subsequent IX metabolite 8-PN.20 An investigation of the pharmacokinetics of pure IX in rats confirmed in vivo formation of 8-PN from IX.24 Detailed metabolism studies have demonstrated that P450s, mainly P4501A2, as well as gut microbiota can metabolize IX to 8-PN (Figure 1).25,26 Eubacterium limosum is the gut bacterium mainly responsible for this metabolism. It is present in the colon of many humans.26 As the concentration of E. limosum varies strongly between individuals, the final in vivo concentration of the phytoestrogen 8-PN may also differ significantly in a diverse population.26 While the metabolism of XH to DMX by P450 or microbiota is theoretically possible (Figure 1), it might not be clinically relevant. DMX would quickly cyclize to both 8-PN and 6-PN.16 After oral administration of purified XH, no 6-PN has been observed in human subjects, indicating that O- demethylation of XH and cyclization to 6-PN is not a major metabolic pathway in humans.20 A recent pharmacokinetic study in healthy women and men administering a single dose of 500 mg of pure 6-PN or 8-PN revealed that 8-PN plasma levels were significantly higher than 6-PN plasma concentrations.27
4. ESTROGEN RECEPTOR (ER) AS A BIOLOGICAL TARGET
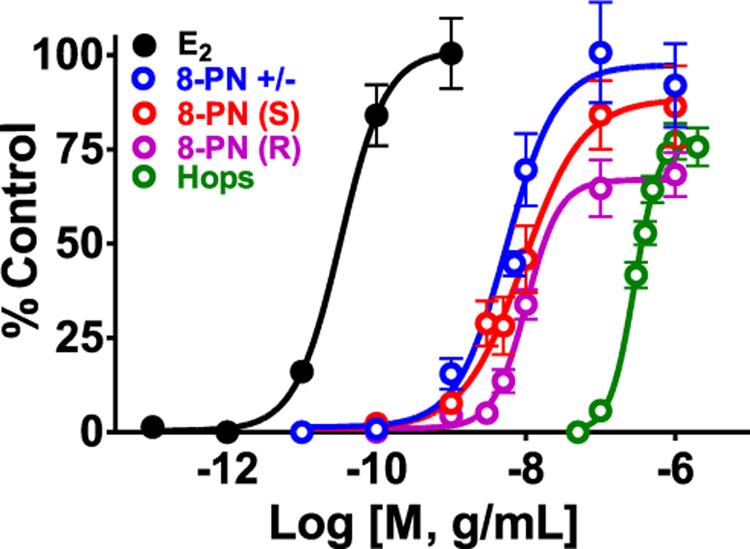
Hops contain the potent phytoestrogen 8-PN, which binds tighter to ERα compared to ERβ.28,29 In an animal model of menopausal hot flashes, 8-PN, similar to estradiol, restored the normal tail skin temperature of ovariectomized animals.30 This suggests that 8-PN may also lead to menopausal symptom relief in women.5,31 8-PN’s concentration in crude plant extracts is at least 10 times lower than XH;32 however, in vivo, more 8-PN is likely formed from XH (Figure 1). Also, the concentration in hormone sensitive tissues (e.g., breast) could be considerably higher due to active transport mediated by ERα.33 In several hormone responsive cell-based assays (Figure 2) as well as animal model studies, 8-PN exhibited estrogenic activity.28,34–36 Using the procedure of Pisha et al., as shown in Figure 2, the racemic mixture of the R/S forms of 8-PN had the highest estrogenic activity.37 8-PN is in part synthesized from IX in vivo (Figure 1) and it has been shown that R- and S-8-PN can be formed from IX in vivo.24 An animal study described that while a standardized extract of spent hops containing 8-PN did not increase uterine weight in a rat model, pure 8-PN did increase uterine weight and the height of luminal epithelial cells.34,38 The prenyl group in 8-PN is likely responsible for its high ERα potency and efficacy, since naringenin lacks the 8-prenyl side chain and is much less potent than 8-PN.39 Prenylation increases the hydrophobicity of 8-PN, which optimizes binding in the hydrophobic pocket of ERα. Prenylation also enhances the PK profile of 8-PN as discussed above. In ovariectomized rat models, a standardized spent hop extract did not show estrogenic effects on the uterus and on the mammary gland.40,41 However, weak bone protective effects on bone loss occurred after 8 weeks of treatment with the standardized hop extract (60 mg/kg BW/d) indicating beneficial estrogenic activity.40
Figure 2.
Estrogenicity of 8-PN enantiomers compared with estradiol and the standardized spent hop extract. ERα endometrial carcinoma cells (Ishikawa) were grown in 96 well plate (5 × 104 cells/well) in estrogen-free media for 24 h. Subsequently, they were treated with the spent hop extract and compounds at varied concentrations for 96 h at 37 °C, then washed and lysed. Induced estrogen-dependent alkaline phosphatase activity was evaluated spectrophotometrically at 405 nm by the absorbance of the formed p-nitrophenol from the p-nitrophenol phosphate substrate (1 mg/mL). Estrogenic activity of respective treatments was assessed by comparison to the estradiol control (1 nM). The results represent the mean ± SD of at least three independent experiments in triplicate for each sample. The dose response curves were generated using GraphPad Prism.
5. AROMATASE AS A BIOLOGICAL TARGET (FIGURE 3)
Figure 3.
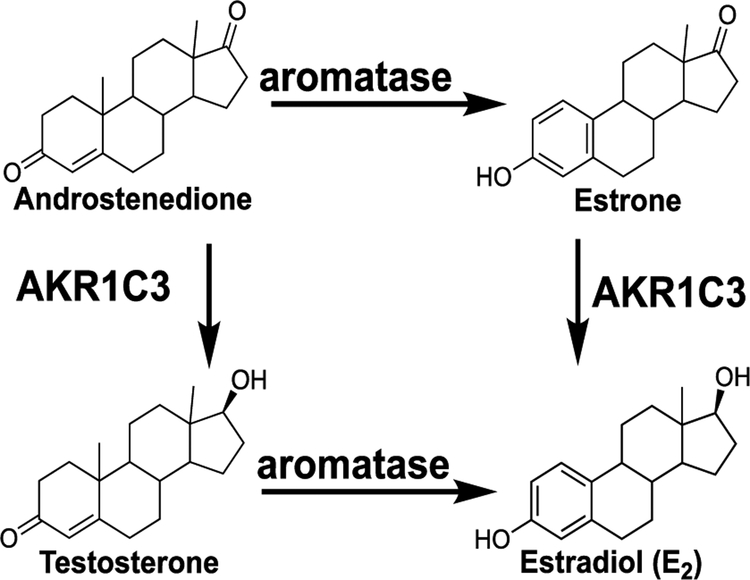
Formation of estrone/estradiol catalyzed by aromatase and aldoketoreductase 1C3 (AKR1C3).
Aromatase is the rate-limiting enzyme in the generation of estradiol/estrone in the mammary gland (Figure 3). As a result, inhibiting aromatase with dietary supplements can serve as a chemopreventive mechanism particularly in obese menopausal women.42–44 Previous work has shown that the phytoestrogen, 8-PN, is a potent inhibitor of aromatase with an IC50 = 0.08 μM.45,46 This value is in the same range as our data, which gave an approximate IC50 of 0.06 μM (Figure 4). As shown in Figure 4, the spent hop extract had similar activity to the positive control ketoconazole. XH and 6-PN were also tested, and they were weak aromatase inhibitors. It has been speculated that the potent inhibitor activity of 8-PN might be due to its structural similarity to both the substrates and products of the aromatase reaction (Figure 3).45,47 Finally, different kinds of beer have also been shown to inhibit aromatase in choriocarcinoma derived JAR cells.46
Figure 4.
Inhibition of aromatase by hops and hop compounds. Aromatase activity was determined using the CYP19/MFC High- Throughput Inhibitor Screening Kit according to the manufacturer’s protocol (Corning, Woburn, MA). The NADPH-cofactor mix was prepared and added to the 96-well plate. The standardized spent hop extract, the hop compounds, and the positive control, ketoconazole, were dissolved in acetonitrile, added to the 96-well plate, and preincubated for 10 min after serial dilutions were performed. The compounds were then incubated with the enzyme/substrate mix (CYP19/7-methoxy-4-trifluoromethyl coumarin) for 30 min. The stop reagent was added thereafter, and the fluorescent signal was read at an excitation of 409 nm and emission of 530 nm. The data represent the average ± SD of three experiments. The dose response curves were generated using GraphPad Prism.
6. THE ARYL HYDROCARBON RECEPTOR (AHR) AS A BIOLOGICAL TARGET (FIGURE 5)
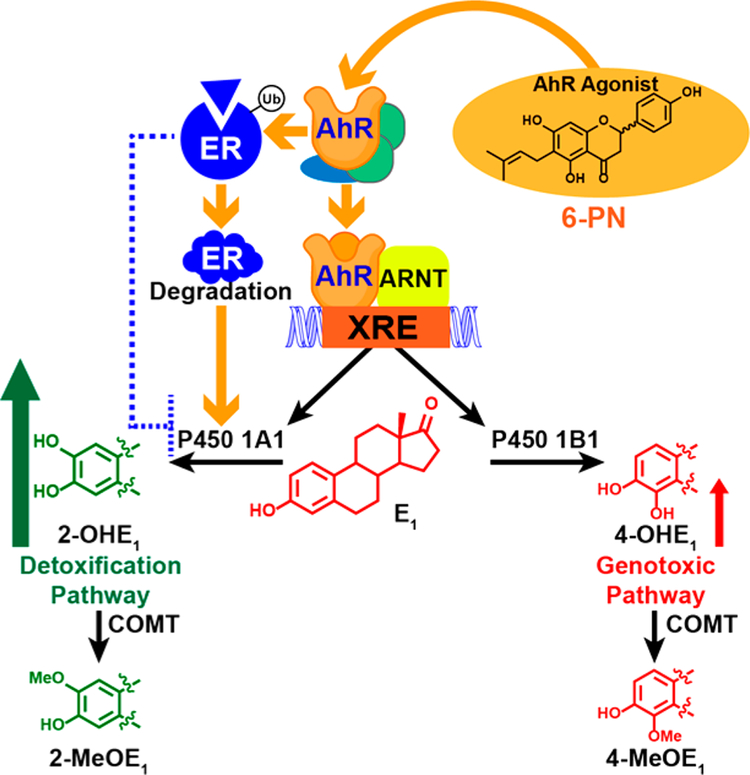
Figure 5.
6-PN, an AhR agonist leading to the induction of P450 1A1. 6-PN increases estrogen metabolism after AhR-mediated upregulation of estrogen metabolizing enzymes, P450 1A1 and P450 1B1. AhR induction can be regarded as an antiestrogenic mechanism, since P450 1A1 and P450 1B1 induce 2- and 4-hydroxylation of estrogens and thus limit E2 concentrations and AhR activation mediates ubiquitination and degradation of ERα.48
Hops may influence not only estrogen synthesis but also estrogen metabolism through the AhR pathway. It has been shown that a standardized spent hop extract21 can alter P450 1A1/1B1-mediated estrogen metabolism in malignant (MCF-7) and nontumorigenic mammary epithelial cells (MCF-10A).48 The relative levels of the estrogen metabolites, 2-methoxyestrone (2-MeOE1) and 4-methoxyestrone (4-MeOE1), generated via the detoxification and genotoxic pathways, respectively, were studied via LC-MS/MS analysis (Figure 5). These metabolites are stable biomarkers that reflect levels of the catechols, 2-OHE1 and 4-OHE1, which are produced by P450 1A1 and P450 1B1, respectively. Induction of the 2-hydroxylation pathway occurred in MCF-10A cells with little effect on the genotoxic 4-hydroxylation pathway.48 Significant CYP1A1 induction was detected by RT-qPCR with only slight increases in CYP1B1 expression in both MCF-10A and MCF-7 cells. An analysis of the bioactive compounds showed that 6-PN was the most potent activator of AhR signaling, which induces CYP1A1 and CYP1B1 genes, although 8-PN did show some activity in MCF-7 cells.48 Since ER is known to selectively suppress CYP1A1 expression, 6-PN, similar to other AhR agonists, likely also targets ER for proteasomal degradation, which leads to substantial CYP1A1 induction (Figure 5).49
7. REACTIVE OXYGEN SPECIES AS A BIOLOGICAL TARGET
Hops contain many prenylated aromatic compounds, such as 8-PN and XH, that can act as scavengers of reactive oxygen species (ROS) and prevent oxidative damage to proteins and DNA.50–53 The data suggest that the prenylated phenols in particular can protect cellular proteins such as low-density lipoproteins (LDL) from oxidation.53 Similar protective effects on DNA oxidation have been reported, especially for XH.54,55 XH could be an indirect antioxidant by increasing GSH. A small clinical study where participants consumed a drink containing XH (12 mg/day) showed a significant drop in 8-oxo-2′- deoxyguanosine (8-oxo-dG) levels, a biomarker of hydroxy radical induced oxidative DNA damage.56 Beer has also been shown to protect DNA from oxidative damage and the amount of protection correlated with the content of phenolic constituents.57 Finally, the antioxidant activities of a spent hop extract and the isolated hop compounds were measured according to their relative ability to scavenge DPPH free radicals.55 The data showed that the hop extract and compounds had weak radical scavenging activities, which suggests other biological targets might be more important. At higher concentrations, pro-oxidant activities have also been reported for XH. In a study using breast cancer cells (MCF-7), XH decreased ROS production at low concentrations; however, XH increased ROS at higher concentrations.51
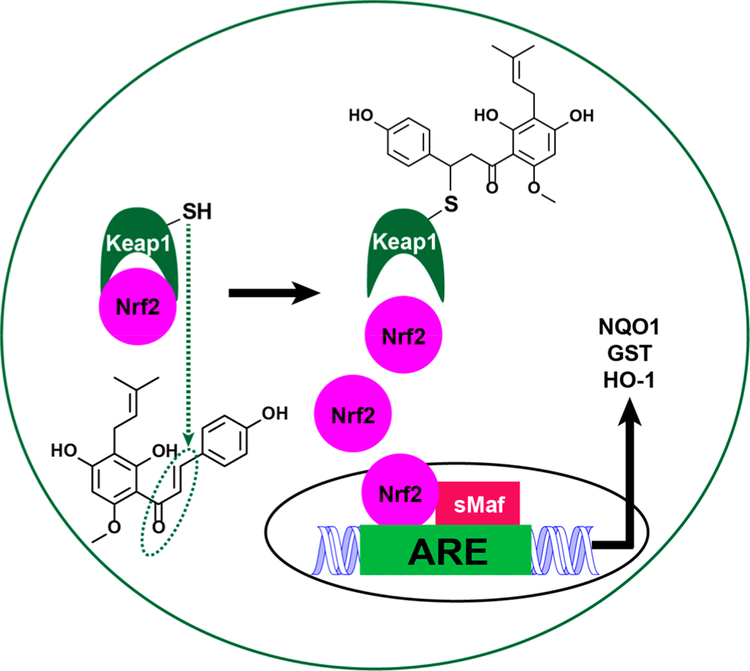
8. KEAP1-NRF2 AS A BIOLOGICAL TARGET (FIGURE 6)
Figure 6.
XH alkylation of cysteine residues in Keap1 leading to activation and translocation of Nrf2 to the nucleus inducing the synthesis of detoxification enzymes such as NQO1, HO-1, and GST.33,55
XH has demonstrated various chemopreventive properties. For example, alkylation of Keap1 by electrophiles such as the α,β- unsaturated carbonyl on XH (Figure 1) causes accumulation of Nrf2 in the nucleus where it synthesizes detoxification enzymes such as NQO1, heme oxygenase-1 (HO-1), and GST (Figure 6).33,55,58,59 For example, it has been shown that pretreatment of rat derived pheochromocytoma (PC12) cells with XH at submicromolar concentrations upregulated a panel of detoxification genes as well as the corresponding gene products, including GSH, HO-1, NQO1, thioredoxin, and thioredoxin reductase.52 Keap1 has been identified as a target protein of XH using a click chemistry approach as well as using a LC-MS/MS assay.60,61 A spent hop extract and XH have been demonstrated to induce the activity of NQO1 in vitro and in vivo animal models.33 Induction of NQO1 by XH has been shown to reduce menadione induced DNA damage in vitro.55 Similarly, XH protected DNA against the genotoxicity of 2-amino-3-methylimidazo[4,5-f]quinoline (IQ), benzo(a)- pyrene, and oxidative DNA damage in HepG2 cells, rat liver slices, and in vivo.62–65 In a human intervention trial where 22 subjects drank a drink containing 12 mg of XH, lymphocytes were collected and incubated with carcinogens and human liver homogenate (S9). The data showed a significant reduction in DNA damage and a clear induction of α-GST (43%), suggesting the Keap1-Nrf2 pathway was involved.66
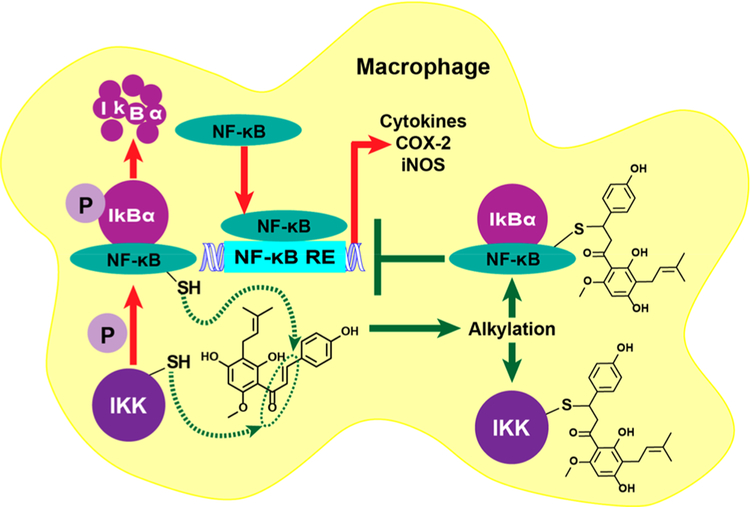
9. IKK/NF-κB AS A BIOLOGICAL TARGET (FIGURE 7)
Figure 7.
XH alkylation of IKK and NF-κB cysteine residues. IκBα sequesters NF-κB in the cytoplasm, yet inflammatory stimuli activate IKK phosphorylation of IκBα and subsequent IκBα degradation. This allows NF-κB to translocate to the nucleus and activate inflammatory genes, such as iNOS and COX-2. XH is anti-inflammatory, since it alkylates cysteine residues in IKK, which prevents degradation of IκBα, and cysteine residues in NF-κB, which prevents NF-κB DNA binding.70
Another cytoprotective pathway of XH is the IKK/NF-κB pathway, which leads to anti-inflammatory activities. NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) regulates genes involved in the formation of pro-inflammatory factors, such as cytokines, inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2; Figure 7). As a result, inhibition of NF-κB activation is a key chemopreventive target.3,67 XH has been shown to downregulate both constitutive and inducible NF-κB activation.3,68,69 The mechanism likely involves modification of the cysteine residues by XH in IκBα kinase (IKK) and/or NF-κB, which directly leads to suppression of NF-κB regulated gene products and potentiation of apoptosis in human leukemia and myeloma cells (Figure 7).70 The presence of reducing agents reversed the effect of XH on IKK, suggesting a role for cysteine residues.70 In support of this, XH had no effect on the IKK activity when cysteine residue 179 of IKK was mutated to alanine and DNA binding of the p65 subunit of NF-κB was inhibited when cysteine residue 38 was mutated to serine.69,70 Further evidence from our laboratory has shown that a standardized spent hop extract and XH can downregulate NF-κB luciferase in MCF-7 cells (Figure 8) and inhibit iNOS activity in RAW 264.7 cells (Figure 9). Similar data were obtained previously, which showed XH downregulated TNFα-induced iNOS activity.10 Additionally, XH inhibits the signal transducer and activator of transcription 3 (STAT3) and activators of this pathway (e.g., IL-6), which leads to reduced inflammatory response, survival, migration, and angiogenesis.71 The mechanism reportedly involves XH interfering with LPS receptor binding. In summary, several lines of evidence suggest that XH-mediated inhibition of pro-inflammatory biological targets can play a key role in inhibiting carcinogenesis.71
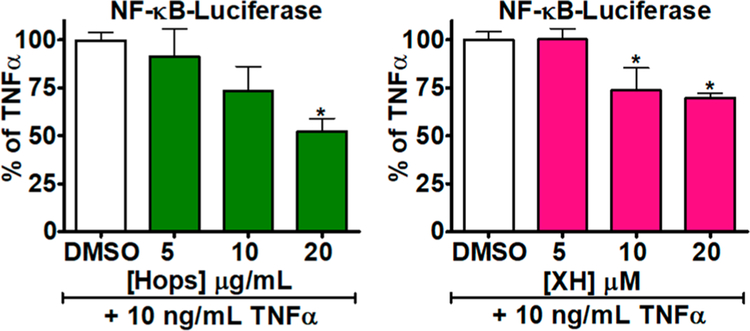
Figure 8.
Hops and XH inhibition of NF-kB luciferase. MCF-7 cells were grown in six-well plates (25 × 104 cells/well) for 24 h in estrogen-free media at 37 °C. After 24 h, cells were transfected with the NF-κB-luciferase reporter plasmid and treated with TNF-α (10 ng/mL) and (A) the standardized spent hop extract or (B) XH for 6 h before lysis with passive lysis buffer. NF-κB-luciferase activity was measured using the Promega Dual-Luciferase Reporter Assay System (Madison, WI). Results represent the mean ± SD of three independent experiments analyzed by one-way ANOVA with Dunnett’s multiple comparison post-test; *p < 0.001.
Figure 9.
Hops and XH inhibition of iNOS. RAW 264.7 cells were plated at a density of 12 × 104 cells/mL in 96-well plates and incubated at 37 °C for 24 h. Cells were treated with LPS (1 μg/mL) and (A) the standardized spent hop extract or (B) XH and incubated at 37 °C for 24 h. Griess reagent (150 μL of 0.5% sulfanilamide and 0.05% (N-1-naphthyl) ethylenediamine dihydrochloride in 2.5% w/w H3PO4) was added to a 96-well plate containing media collected from cells (50 μL). Nitrite levels were detected after the plate was incubated at RT for 30 min. Absorbance was measured at 530 nm, and concentrations were calculated using a NaNO2 standard curve. Results represent the mean ± SD of three independent experiments and are analyzed by one-way ANOVA with Dunnett’s multiple comparison post-test; *p < 0.001.
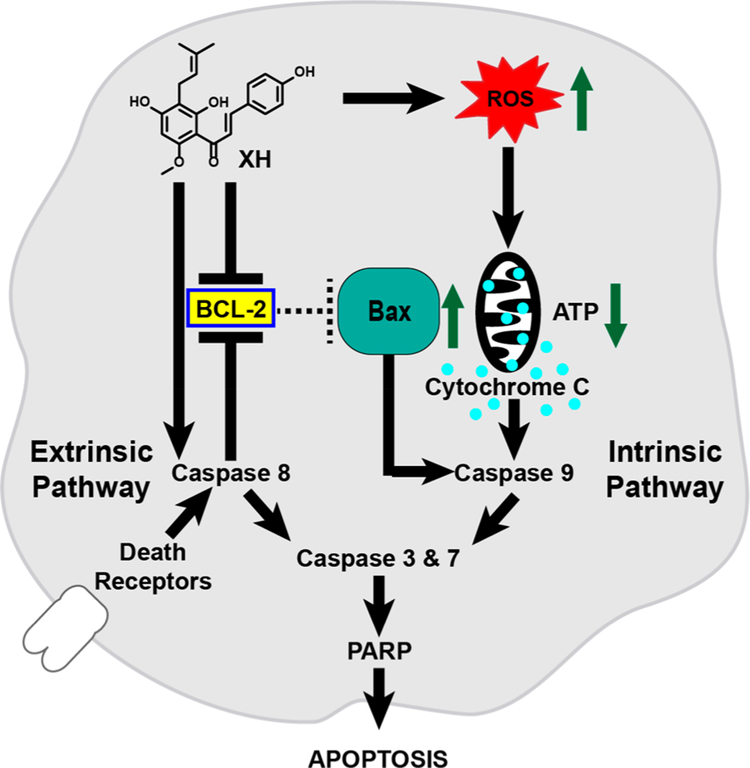
10. XH TARGETS BCL-2 AND MITOCHONDRIA LEADING TO APOPTOSIS (FIGURE 10)
Figure 10.
XH induction of apoptosis at high concentrations. XH treatment initiates intrinsic and extrinsic apoptosis pathways by multiple mechanisms, including downregulation of antiapoptotic (BCL-2) and induction of pro-apoptotic (Bax) proteins, increases in ROS, leading to decreased mitochondrial membrane potential and subsequent cytochrome C release, and activation of caspases. As a result, cleavage of PARP and reduced proliferation of tumor cells has been observed after XH treatments.72–75
While XH induces cytoprotective mechanism at low concentrations, it leads to apoptosis and cell cytotoxicity at higher concentrations.51 The apoptosis inducing and cell proliferation inhibiting properties by XH have been demonstrated in several cell lines as well as in animal tumor models.7,72–76 Different pathways have been described for XH that lead ultimately to apoptosis and reduction of cell survival.73–76 For example, XH (15 μM for 48 h) has been demonstrated to activate caspases of the extrinsic (caspase 8) and intrinsic pathway (caspase 9) in human colon cancer cells, resulting in downstream activation of caspase 3 and cleavage of Poly(ADP-ribose)polymerase (PARP), as the hallmark of apoptosis (Figure 10).74 Downregulation of the antiapoptotic protein B-cell lymphoma 2 (Bcl-2) and upregulation of the pro-apoptotic protein Bcl-2-associated X protein (Bax) contributed to activation of the intrinsic apoptosis pathway.74 In a different study, induction of oxidative stress mainly through superoxide formation in mitochondria has been reported as a cause for apoptosis induction by XH.73 Further analysis showed a decrease in mitochondrial electron flux and intracellular ATP levels by XH (around 25 μM), leading to cytochrome c release and ultimately to apoptosis (Figure 10). Similarly, in an analysis in adipocytes, XH increased ROS, resulting in a lower mitochondrial membrane potential, cytochrome C release, and induction of apoptosis.72 As mentioned above (see section 6, Reactive Oxygen Species as a Biological Target), at low concentrations, XH decreased ROS production in the breast cancer cell line, MCF-7, while XH increased ROS and reduced cell viability at higher con- centrations.51 This is in line with other studies reporting that the Michael acceptor, XH, reduced oxidative stress and inflammation at low concentrations and leads to a decrease of cell proliferation at higher concentrations.9,55,77 As an example, XH has also been reported to decrease biomarkers of apoptosis and oxidative stress in an aged rat model of increased apoptosis, oxidative stress, and inflammation.78
Studies of XH-rich hop extracts analyzing the influence on apoptosis remain preliminary. In vitro studies in endometrial cancer cells (Ishikawa) and mouse hepatoma cells (Hepa1c1c7) have shown that cell survival after treatment with various spent hop extracts is directly correlated with the XH concentration.8 NQO1 induction properties and estrogenic activities of these spent hop extracts were observed at much lower concentrations than a decrease in cell survival.8 Overall, these results may indicate that XH in hop products may more likely protect against oxidative stress in vivo, as XH concentrations that lead to apoptosis may be more difficult to reach after administration of clinical doses of (spent) hop extracts.
11. AMP-ACTIVATED PROTEIN KINASE SIGNALING PATHWAY (AMPK) AS A BIOLOGICAL TARGET OF XH TO REGULATE LIPID AND GLUCOSE METABOLISM (FIGURE 11)
Figure 11.
XH influence on lipid and glucose metabolism. XH exhibits antiobesity and antiglycemic effects by modulating the AMPK pathway. XH increases ROS, which can directly activate AMPK or decrease mitochondrial coupling, resulting in an increased AMP/ATP ratio, and in turn activate the AMPK pathway. AMPK phosphorylates multiple targets, including PPAR-α and SREBP, which results in increased β-oxidation and lipolysis and decreased fatty acid synthesis and adipogenesis.80–82
Different in vitro and in vivo studies also demonstrated antiobesity and antiglycemic effects for XH.79–82 Similarly, in a male rodent obesity model, an XH-rich hop extract significantly decreased body weight as well as liver triacylglycerol and cholesterol values compared to the high fat diet.83 XH’s influence on body weight, lipid metabolism, and glucose levels has been reported to be mediated through multiple biological targets.79–82 An increase of lipolysis and an accelerated break down of fatty acids through enhanced beta oxidation (Figure 11) has been described in adipocytes81 and in obesity animal models after treatment with XH.82 Beta oxidation is likely increased by XH through activation of the AMPK signaling pathway81 and subsequent increase of peroxisome proliferator-activated receptor α (PPARα) mRNA expression (Figure 11).82
AMPK is the principal sensor of cellular energy status and is activated by an increase of AMP/ATP ratio and ROS.84,85 The AMP/ATP ratio can be enhanced through mitochondrial impairment. Mitochondrial uncoupling (electron transport that is not used to drive ATP synthesis) induced by XH-mediated short-term ROS formation has been demonstrated to increase AMP-activated protein kinase signaling pathways,80 leading to increased catabolism and higher energy expenditure (Figure 11).81 An elevation of the AMPK signaling pathway by XH also led to downregulation of sterol regulatory element-binding protein-lc (SREBP-lc) mRNA expression in an rodent obesity model, consequently reducing fatty acid synthesis and adipogenesis.82
In addition, XH has been reported to increase beige-ing of white adipocytes, which involves accelerated thermoregulation and lipolysis contributing to XH’s antiobesity effects.81 A char-acteristic of beige adipocytes is enhanced mitochondrial biogenesis and thus higher energy expenditure. XH markedly increased mitochondrial content and markers of mitochondrial biogenesis in adipocytes, such as PGC-lα.81 XH’s influence on mitochondria and oxidative stress followed by AMPK signaling pathway induction may in part explain the observed antiobesity activity of XH in rodent obesity studies.80 Interestingly, impaired energy metabolism is also a primary defect in type 2 diabetes, and two current diabetic therapeutics have been shown to act via AMPK activation.85 As a result, stimulation of AMPK by XH might also explain some of its antiglycemic effects (Figure 11).86
Other biological targets for XH’s influence on lipid metabolism have been described. For example, XH has been reported to inhibit rat liver diacylglycerol acyltransferase and triglyceride transport, as well as to inhibit cholesteryl ester transfer protein activity and to induce apoptosis of mature adipocytes.79 While different rodent obesity models in male animals reported antiobesity and antiglycemic effects after XH treatment, these activities were not reported in a similar model in female rats.86 Additional studies are needed to elucidate the difference of XH treatment on sex-specific characteristics of lipid metabolism. Overall, available data suggest a regulating influence of XH and XH-rich hop extracts on lipid metabolism at least in male animals and warrants further analysis in clinical studies.
12. AMP-ACTIVATED PROTEIN KINASE SIGNALING PATHWAY (AMPK) AS A BIOLOGICAL TARGET OF XH TO REDUCE ANGIOGENESIS
The AMPK pathway seems also to be involved in XH’s antiangiogenic properties.87 XH showed antiangiogenic effects in in vitro and in vivo models.68,87,88 Treatment of endothelial cells with XH increased AMPK activity, which likely resulted in antiangiogenic effects. XH-mediated AMPK activation resulted in a decrease of endothelial NOS phosphorylation, ultimately reducing NO levels and thus reducing angiogenesis. In addition, XH’s antiangiogenic activity has also been shown to be mediated by inhibition of AKT and other pathways.68,87 XH-rich hop extracts have not yet been well analyzed for its antiangiogenic activity; therefore, additional studies are warranted to elucidate the overall antiangiogenic properties of hop extracts.
13. INHIBITION OF P450S AS A BIOLOGICAL TARGET
Finally, the hop compounds have been reported to inhibit several P450 enzymes.89–91 A number of different beers have also been tested with significant inhibition observed with various P450s particularly with the dark porter style beers.92,93 At 10 μM, XH almost completely inhibited the 7-ethoxyresorufin O-deethylase activity of P450 1A1 and P450 1B1.89 In contrast, P450 1A2 was inhibited by 8-PN and IX (>90% inhibition, 10 μM). P450 3A4 and P450 2E1 were not inhibited by the hop compounds, which shows they are selective inhibitors of P450s.89 The standardized spent hop extract at 5 μg/mL showed significant inhibition of P450 2C8 and P450 2C9.90 IX was the most potent inhibitor of P450 2C8, whereas 8-PN had highest inhibitory activity for P450 2C9.90 Whether these hop flavonoids would lead to P450 inhibition after administration of the standardized spent hop extract in humans is currently under analysis in a phase I study in our Botanical Center.
14. DESIGNER HOP EXTRACTS SHOWED SIMULTANEOUS MODIFICATION OF TWO BIOLOGICAL TARGETS (FIGURE 12)
Figure 12.
Designer extracts. Two spent hop extracts were designed with different concentrations of 8-PN. One extract with higher 8-PN concentrations modulates estrogenic effects in postmenopausal women, yet retains chemopreventive effects derived from XH. The second extract designated for use by younger women (premenopausal extract) contains lower concentrations of 8-PN than the postmenopausal extract but similar XH concentrations with potential chemopreventive properties.8
We recently have shown that hop extracts can be modified to selectively increase or decrease the concentrations of certain bioactive compounds in hops.8 For example, all populations of women would benefit from the chemopreventive compounds such as XH; however, premenopausal women are unlikely to benefit from the estrogenic constituent 8-PN. Through a series of differential liquid-only chromatographic steps, two spent hop extracts were created: one designated for premenopausal women with significant amounts of XH and very little 8-PN, and one for postmenopausal women with XH and 8-PN for menopausal symptom relief (Figure 12). These data show that once the bioactive compounds have been identified in extracts, DESIGNER (Deplete and Enrich Select Ingredients to Generate Normalized Extract Resources) extracts could be created to maximize beneficial outcomes and limit potential unwanted effects.
15. CONCLUSIONS AND FUTURE DIRECTIONS
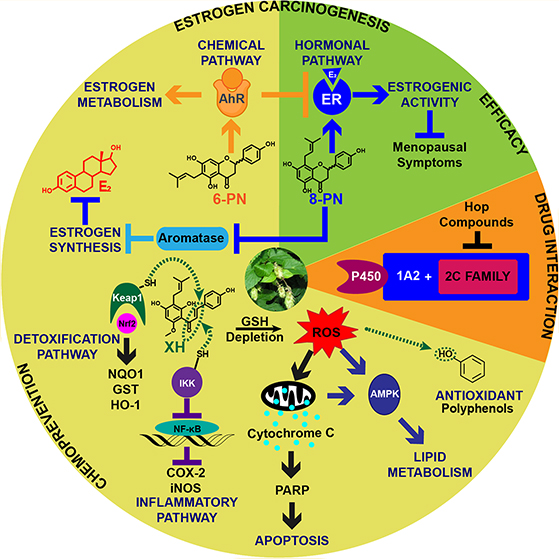
In conclusion, accumulating data are showing that spent hop extracts have multiple biological targets, leading to polypharmacological outcomes in vivo (Figure 13). Estrogenic effects associated with hops are most likely due to 8-PN, a potent ER ligand. This phytoestrogen is also an aromatase inhibitor that could protect women from estrogen carcinogenesis. The prenylated phenols are weak scavengers of ROS; however, these compounds might function indirectly as antioxidants through an increase of GSH synthesis and other antioxidative enzymes. As a Michael acceptor, XH is a Keap1-Nrf2 activator, leading to induction of detoxification enzymes such as NQO1 and GST. XH also alkylates IKK and NF-κB, resulting in anti-inflammatory activity. At higher concentrations, XH can have pro-oxidant effects leading to apoptosis and a decrease of cell proliferation. Therefore, XH concentrations in the high nanomolar to low micromolar range would be ideal to achieve XH’s cytoprotective effect in vivo. Finally, XH has been described to activate AMPK, which is at least in part responsible for XH’s antiobesity and antiglycemic effects. Hops also contain 6-PN, a potent agonist of AhR, leading to selective upregulation of the P450 1A1-mediated estrogen detoxification pathway. The hop compounds are also selective inhibitors of various P450s including P450 1A1, 1A2, 1B1, 2C8, 2C9, and 2C19. Finally, experiments with designer extracts have shown that spent hop extracts can be prepared which contain tailored doses of desirable bioactive compounds while limiting exposure to compounds that might be associated with adverse effects. Understanding the mechanism of action and biological targets of bioactive compounds in hop dietary supplements used for women’s health will ultimately lead to standardized botanical products with higher efficacy and safety and help optimize the chemopreventive properties and other health benefits of hops.
Figure 13.
Summary of biological targets of spent hop extracts.
ACKNOWLEDGMENTS
This work as well as cited work from the authors’ laboratories were supported by NIH grants P50 AT000155 (UIC/NIH Botanical Center) and U41 AT008706 (CENAPT).
ABBREVIATIONS
- AKR1C3
aldoketoreductase 1C3
- AMPK
AMP-activated protein kinase
- ARE
antioxidant response element
- AhR
aryl hydrocarbon receptor
- Bax
pro-apoptotic protein Bcl-2- associated X protein
- Bcl-2
B-cell lymphoma 2
- COX-2
cyclooxygenase-2
- DMX
desmethylxanthohumol
- ER
estrogen receptor
- GST
glutathione-S-transferase
- GSH
glutathione
- HO-1
heme oxygenase-1
- IKK
inhibitor of kappa B protein kinase
- iNOS
inducible nitric oxide synthase
- IX
isoxanthohumol
- Keap1
Kelch like-ECH-associated protein 1
- 2-MeOE1
2-methoxyestrone
- 4-MeOE1
4-methoxyestrone
- NQO1
NAD(P)H-quinone oxidoreductase 1
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- Nrf2
nuclear factor (erythroid-derived 2)-like 2
- PPARα
peroxisome proliferator-activated receptor α
- PARP
poly(ADP-ribose)- polymerase
- 6-PN
6-prenylnaringenin
- 8-PN
8-prenylnaringe- nin
- ROS
reactive oxygen species
- SREBP-1c
sterol regulatory element-binding protein-1c
- SFE
supercritical fluid extraction
- TNFα
tumor necrosis factor alpha
- XH
xanthohumol
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Stevens JF, and Page JE (2004) Xanthohumol and related prenylflavonoids from hops and beer: to your good health! Phytochemistry 65, 1317–1330. [DOI] [PubMed] [Google Scholar]
- (2).Karabin M, Hudcova T, Jelinek L, and Dostalek P (2016) Biologically active compounds from hops and prospects for their use. Compr. Rev. Food Sci. Food Saf 15, 542–567. [DOI] [PubMed] [Google Scholar]
- (3).Karabin M, Hudcova T, Jelinek L, and Dostalek P (2015) Biotransformations and biological activities of hop flavonoids. Biotechnol. Adv. 33, 1063–1090. [DOI] [PubMed] [Google Scholar]
- (4).Aghamiri V, Mirghafourvand M, Mohammad-Alizadeh-Charandabi S, and Nazemiyeh H (2016) The effect of Hop (Humulus lupulus L.) on early menopausal symptoms and hot flashes: A randomized placebo-controlled trial. Complement. Ther. Clin. Pract 23, 130–135. [DOI] [PubMed] [Google Scholar]
- (5).Dietz BM, Hajirahimkhan A, Dunlap TL, and Bolton JL (2016) Botanicals and their bioactive phytochemicals for women’s health. Pharmacol. Rev 68, 1026–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Keiler AM, Zierau O, and Kretzschmar G (2013) Hop extracts and hop substances in treatment of menopausal complaints. Planta Med. 79, 576–579. [DOI] [PubMed] [Google Scholar]
- (7).Venturelli S, Burkard M, Biendl M, Lauer UM, Frank J, and Busch C (2016) Prenylated chalcones and flavonoids for the prevention and treatment of cancer. Nutrition 32, 1171–1178. [DOI] [PubMed] [Google Scholar]
- (8).Dietz BM, Chen SN, Alvarenga RFR Dong H, Nikolic D, Biendl M, van Breemen RB, Bolton JL, and Pauli GF (2017) DESIGNER extracts as tools to balance estrogenic and chemopreventive activities of botanicals for women’s health. J. Nat. Prod 80, 2284–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Gerhauser C, Alt A, Heiss E, Gamal-Eldeen A, Klimo K, Knauft J, Neumann I, Scherf HR, Frank N, Bartsch H, and Becker H (2002) Cancer chemopreventive activity of xanthohumol, a natural product derived from hop. Mol. Cancer Ther 1, 959–969. [PubMed] [Google Scholar]
- (10).Cho YC, Kim HJ, Kim YJ, Lee KY, Choi HJ, Lee IS, and Kang BY (2008) Differential anti-inflammatory pathway by xanthohumol in IFN-gamma and LPS-activated macrophages. Int. Immunopharmacol 8, 567–573. [DOI] [PubMed] [Google Scholar]
- (11).Chadwick LR, Nikolic D, Burdette JE, Overk CR, Bolton JL, van Breemen RB, Fröhlich R, Fong HH, Farnsworth NR, and Pauli GF (2004) Estrogens and congeners from spent hops (Humulus lupulus). J. Nat. Prod 67, 2024–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Chadwick LR, Pauli GF, and Farnsworth NR (2006) The pharmacognosy of Humulus lupulus L. (hops) with an emphasis on estrogenic properties. Phytomedicine 13, 119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Kötter U, and Biendl M (2010) Hops (Humulus lupulus): A Review of its Historic and Medicinal Uses. HerbalGram 87, 44–57. [Google Scholar]
- (14).Dresel M, Dunkel A, and Hofmann T (2015) Sensomics analysis of key bitter compounds in the hard resin of hops (Humulus lupulus L.) and their contribution to the bitter profile of Pilsner-type beer. J. Agric. Food Chem 63, 3402–3418. [DOI] [PubMed] [Google Scholar]
- (15).Milligan S, Kalita J, Pocock V, Heyerick A, De Cooman L, Rong H, and De Keukeleire D (2002) Oestrogenic activity of the hop phyto-oestrogen, 8-prenylnaringenin. Reproduction 123, 235–242. [PubMed] [Google Scholar]
- (16).Chen SN, Lankin DC, Chadwick LR, Jaki BU, and Pauli GF (2009) Dynamic residual complexity of natural products by qHNMR: solution stability of desmethylxanthohumol. Planta Med. 75, 757–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Nikolic D, Li Y, Chadwick LR, and van Breemen RB (2006) In vitro studies of intestinal permeability and hepatic and intestinal metabolism of 8-prenylnaringenin, a potent phytoestrogen from hops (Humulus lupulus L.). Pharm. Res 23, 864–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Pang Y, Nikolic D, Zhu D, Chadwick LR, Pauli GF, Farnsworth NR, and van Breemen RB (2007) Binding of the hop (Humulus lupulus L.) chalcone xanthohumol to cytosolic proteins in Caco-2 intestinal epithelial cells. Mol. Nutr. Food Res 51, 872–879. [DOI] [PubMed] [Google Scholar]
- (19).Yilmazer M, Stevens JF, and Buhler DR (2001) In vitro glucuronidation of xanthohumol, a flavonoid in hop and beer, by rat and human liver microsomes. FEBS Lett. 491, 252–256. [DOI] [PubMed] [Google Scholar]
- (20).Legette L, Karnpracha C, Reed RL, Choi J, Bobe G, Christensen JM, Rodriguez-Proteau R, Purnell JQ, and Stevens JF (2014) Human pharmacokinetics of xanthohumol, an antihyperglycemic flavonoid from hops. Mol. Nutr. Food Res. 58, 248–255.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).van Breemen RB, Yuan Y, Banuvar S, Shulman LP, Qiu X, Alvarenga RF, Chen SN, Dietz BM, Bolton JL, Pauli GF, Krause E, Viana M, and Nikolic D (2014) Pharmacokinetics of prenylated hop phenols in women following oral administration of a standardized extract of hops. Mol. Nutr. Food Res 58, 1962–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Bolca S, Li J, Nikolic D, Roche N, Blondeel P, Possemiers S, De Keukeleire D, Bracke M, Heyerick A, van Breemen RB, and Depypere H (2010) Disposition of hop prenylflavonoids in human breast tissue. Mol. Nutr. Food Res 54 (Suppl 2), S284–S294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Rad M, Hümpel M, Schaefer O, Schoemaker RC, Schleuning WD, Cohen AF, and Burggraaf J (2006) Pharmacokinetics and systemic endocrine effects of the phytooestrogen 8-prenylnaringenin after single oral doses to postmenopausal women. Br. J. Clin. Pharmacol 62, 288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Martinez SE, and Davies NM (2015) Enantiospecific pharmacokinetics of isoxanthohumol and its metabolite 8-prenylnaringenin in the rat. Mol. Nutr. Food Res 59, 1674–1689. [DOI] [PubMed] [Google Scholar]
- (25).Guo J, Nikolic D, Chadwick LR, Pauli GF, and van Breemen RB (2006) Identification of human hepatic cytochrome P450 enzymes involved in the metabolism of 8-prenylnaringenin and isoxanthohumol from hops (Humulus lupulus L.). Drug Metab. Dispos 34, 1152–1159. [DOI] [PubMed] [Google Scholar]
- (26).Possemiers S, Rabot S, Espin JC, Bruneau A, Philippe C, Gonzalez-Sarrias A, Heyerick A, Tomas-Barberan FA, De Keukeleire D, and Verstraete W (2008) Eubacterium limosum activates isoxanthohumol from hops (Humulus lupulus L.) into the potent phytoestrogen 8-prenylnaringenin in vitro and in rat intestine. J. Nutr 138, 1310–1316. [DOI] [PubMed] [Google Scholar]
- (27).Calvo-Castro LA, Burkard M, Sus N, Scheubeck G, Leischner C, Lauer UM, Bosy-Westphal A, Hund V, Busch C, Venturelli S, and Frank J (2018) The Oral Bioavailability of 8- Prenylnaringenin from Hops (Humulus Lupulus L.) in Healthy Women and Men is Significantly Higher than that of its Positional Isomer 6-Prenylnaringenin in a Randomized Crossover Trial. Mol. Nutr. Food Res 62, No. 1700838. [DOI] [PubMed] [Google Scholar]
- (28).Overk CR, Yao P, Chadwick LR, Nikolic D, Sun Y, Cuendet MA, Deng Y, Hedayat AS, Pauli GF, Farnsworth NR, van Breemen RB, and Bolton JL (2005) Comparison of the in vitro estrogenic activities of compounds from hops (Humulus lupulus) and red clover (Trifolium pratense). J. Agric. Food Chem 53, 6246–6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Helle J, Kraker K, Bader MI, Keiler AM, Zierau O, Vollmer G, Welsh J, and Kretzschmar G (2014) Assessment of the proliferative capacity of the flavanones 8-prenylnaringenin, 6-(1.1- dimethylallyl)naringenin and naringenin in MCF-7 cells and the rat mammary gland. Mol. Cell. Endocrinol 392, 125–135. [DOI] [PubMed] [Google Scholar]
- (30).Bowe J, Li XF, Kinsey-Jones J, Heyerick A, Brain S, Milligan S, and O’Byrne K (2006) The hop phytoestrogen, 8- prenylnaringenin, reverses the ovariectomy-induced rise in skin temperature in an animal model of menopausal hot flushes. J. Endocrinol 191, 399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Stulikova K, Karabin M, Nespor J, and Dostalek P (2018) Therapeutic perspectives of 8-prenylnaringenin, a potent phytoestrogen from hops. Molecules 23, 660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Nikolic D, and van Breemen RB (2013) Analytical methods for quantitation of prenylated flavonoids from hops. Curr. Anal. Chem 9, 71–85. [PMC free article] [PubMed] [Google Scholar]
- (33).Dietz BM, Hagos GK, Eskra JN, Wijewickrama GT, Anderson JR, Nikolic D, Guo J, Wright B, Chen SN, Pauli GF, van Breemen RB, and Bolton JL (2013) Differential regulation of detoxification enzymes in hepatic and mammary tissue by hops (Humulus lupulus) in vitro and in vivo. Mol. Nutr. Food Res 57, 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Overk CR, Guo J, Chadwick LR, Lantvit DD, Minassi A, Appendino G, Chen SN, Lankin DC, Farnsworth NR, Pauli GF, van Breemen RB, and Bolton JL (2008) In vivo estrogenic comparisons of Trifolium pratense (red clover) Humulus lupulus (hops), and the pure compounds isoxanthohumol and 8- prenylnaringenin. Chem.-Biol Interact 176, 30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Liu J, Burdette JE, Xu H, Gu C, van Breemen RB, Bhat KP, Booth N, Constantinou AI, Pezzuto JM, Fong HH, Farnsworth NR, and Bolton JL (2001) Evaluation of estrogenic activity of plant extracts for the potential treatment of menopausal symptoms. J. Agric. Food Chem 49, 2472–2479. [DOI] [PubMed] [Google Scholar]
- (36).Hajirahimkhan A, Simmler C, Yuan Y, Anderson JR, Chen SN, Nikolic D, Dietz BM, Pauli GF, van Breemen RB, and Bolton JL (2013) Evaluation of estrogenic activity of licorice species in comparison with Hops used in botanicals for menopausal symptoms. PLoS One 8, No. e67947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Pisha E, and Pezzuto JM (1997) Cell-based assay for the determination of estrogenic and anti-estrogenic activities. Methods Cell Sci 19, 37–43. [Google Scholar]
- (38).Diel P, Thomae RB, Caldarelli A, Zierau O, Kolba S, Schmidt S, Schwab P, Metz P, and Vollmer G (2004) Regulation of gene expression by 8-prenylnaringenin in uterus and liver of Wistar rats. Planta Med. 70, 39–44. [DOI] [PubMed] [Google Scholar]
- (39).Kretzschmar G, Zierau O, Wober J, Tischer S, Metz P, and Vollmer G (2010) Prenylation has a compound specific effect on the estrogenicity of naringenin and genistein. J. Steroid Biochem. Mol. Biol 118, 1–6. [DOI] [PubMed] [Google Scholar]
- (40).Keiler AM, Helle J, Bader MI, Ehrhardt T, Nestler K, Kretzschmar G, Bernhardt R, Vollmer G, Nikolic D, Bolton JL, Pauli GF, Chen SN, Dietz BM, van Breemen RB, and Zierau O (2017) A standardized Humulus lupulus (L.) ethanol extract partially prevents ovariectomy-induced bone loss in the rat without induction of adverse effects in the uterus. Phytomedicine 34, 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Keiler AM, Macejova D, Dietz BM, Bolton JL, Pauli GF, Chen SN, van Breemen RB, Nikolic D, Goerl F, Muders MH, Zierau O, and Vollmer G (2017) Evaluation of estrogenic potency of a standardized hops extract on mammary gland biology and on MNU-induced mammary tumor growth in rats. J. Steroid Biochem. Mol. Biol 174, 234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Balunas MJ, Su B, Brueggemeier RW, and Kinghorn AD (2008) Natural products as aromatase inhibitors. Anti-Cancer Agents Med. Chem 8, 646–682. [PMC free article] [PubMed] [Google Scholar]
- (43).Adams LS, and Chen S (2009) Phytochemicals for breast cancer prevention by targeting aromatase. Front. Biosci., Landmark Ed 14, 3846–3863. [DOI] [PubMed] [Google Scholar]
- (44).Nielsen AJ, and McNulty J (2018) Polyphenolic natural products and natural product-inspired steroidal mimics as aromatase inhibitors. Med. Res. Rev, DOI: 10.1002/med.21536. [DOI] [PubMed] [Google Scholar]
- (45).Monteiro R, Faria A, Azevedo I, and Calhau C (2007) Modulation of breast cancer cell survival by aromatase inhibiting hop (Humulus lupulus L.) flavonoids. J. Steroid Biochem. Mol. Biol 105, 124–130. [DOI] [PubMed] [Google Scholar]
- (46).Monteiro R, Becker H, Azevedo I, and Calhau C (2006) Effect of hop (Humulus lupulus L.) flavonoids on aromatase (estrogen synthase) activity. J. Agric. Food Chem 54, 2938–2943. [DOI] [PubMed] [Google Scholar]
- (47).Chen S, Zhang F, Sherman MA, Kijima I, Cho M, Yuan YC, Toma Y, Osawa Y, Zhou D, and Eng ET (2003) Structure-function studies of aromatase and its inhibitors: a progress report. J. Steroid Biochem. Mol. Biol 86, 231–237. [DOI] [PubMed] [Google Scholar]
- (48).Wang S, Dunlap TL, Howell CE, Mbachu OC, Rue EA, Phansalkar R, Chen SN, Pauli GF, Dietz BM, and Bolton JL (2016) Hop (Humulus lupulus L.) extract and 6-prenylnaringenin induce P450 1A1 catalyzed estrogen 2-hydroxylation. Chem. Res. Toxicol 29, 1142–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Marques M, Laflamme L, and Gaudreau L (2013) Estrogen receptor alpha can selectively repress dioxin receptor-mediated gene expression by targeting DNA methylation. Nucleic Acids Res. 41, 8094–8106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Zhang XL, Zhang YD, Wang T, Guo HY, Liu QM, and Su HX (2014) Evaluation on antioxidant effect ofxanthohumol by different antioxidant capacity analytical methods. J. Chem 2014, 249485. [Google Scholar]
- (51).Blanquer-Rossello MM, Oliver J, Valle A, and Roca P (2013) Effect of xanthohumol and 8-prenylnaringenin on MCF-7 breast cancer cells oxidative stress and mitochondrial complexes expression. J. Cell. Biochem 114, 2785–2794. [DOI] [PubMed] [Google Scholar]
- (52).Yao J, Zhang B, Ge C, Peng S, and Fang J (2015) Xanthohumol, a polyphenol chalcone present in hops, activating Nrf2 enzymes to confer protection against oxidative damage in PC12 cells. J. Agric. Food Chem 63, 1521–1531. [DOI] [PubMed] [Google Scholar]
- (53).Miranda CL, Stevens JF, Ivanov V, McCall M, Frei B, Deinzer ML, and Buhler DR (2000) Antioxidant and prooxidant actions of prenylated and nonprenylated chalcones and flavanones in vitro. J. Agric. Food Chem 48, 3876–3884. [DOI] [PubMed] [Google Scholar]
- (54).Carvalho DO, Oliveira R, Johansson B, and Guido LF (2016) Dose-dependent protective and inductive effects of xanthohumol on oxidative DNA damage in Saccharomyces cerevisiae. Food Technol. Biotechnol 54, 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Dietz BM, Kang YH, Liu G, Eggler AL, Yao P, Chadwick LR, Pauli GF, Farnsworth NR, Mesecar AD, van Breemen RB, and Bolton JL (2005) Xanthohumol isolated from Humulus lupulus Inhibits menadione-induced DNA damage through induction of quinone reductase. Chem. Res. Toxicol 18, 1296–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Ferk F, Mišik M, Nersesyan A, Pichler C, Jäger W, Szekeres T, Marculescu R, Poulsen HE, Henriksen T, Bono R, Romanazzi V, Al-Serori H, Biendl M, Wagner KH, Kundi M, and Knasmuller S (2016) Impact of xanthohumol (a prenylated flavonoid from hops) on DNA stability and other health-related biochemical parameters: Results of human intervention trials. Mol. Nutr. Food Res 60, 773–786. [DOI] [PubMed] [Google Scholar]
- (57).Rivero D, Perez-Magarino S, Gonzalez-Sanjose ML, Valls-Belles V, Codoner P, and Muniz P (2005) Inhibition of induced DNA oxidative damage by beers: correlation with the content of polyphenols and melanoidins. J. Agric. Food Chem 53, 3637–3642. [DOI] [PubMed] [Google Scholar]
- (58).Krajka-Kuźniak V, Paluszczak J, and Baer-Dubowska W (2013) Xanthohumol induces phase II enzymes via Nrf2 in human hepatocytes in vitro. Toxicol. In Vitro 27, 149–156. [DOI] [PubMed] [Google Scholar]
- (59).Lee IS, Lim J, Gal J, Kang JC, Kim HJ, Kang BY, and Choi HJ (2011) Anti-inflammatory activity of xanthohumol involves heme oxygenase-1 induction via NRF2-ARE signaling in microglial BV2 cells. Neurochem. Int 58, 153–160. [DOI] [PubMed] [Google Scholar]
- (60).Brodziak-Jarosz L, Fujikawa Y, Pastor-Flores D, Kasikci S, Jirasek P, Pitzl S, Owen R, Klika KD, Gerhauser C, Amslinger S, and Dick TP (2016) A click chemistry approach identifies target proteins of xanthohumol. Mol. Nutr. Food Res 60, 737–748. [DOI] [PubMed] [Google Scholar]
- (61).Liu G, Eggler AL, Dietz BM, Mesecar AD, Bolton JL, Pezzuto JM, and van Breemen RB (2005) Screening method for the discovery of potential cancer chemoprevention agents based on mass spectrometric detection of alkylated Keap1. Anal. Chem 77, 6407–6414. [DOI] [PubMed] [Google Scholar]
- (62).Plazar J, Žegura B, Lah TT, and Filipič M (2007) Protective effects of xanthohumol against the genotoxicity of benzo(a)pyrene (BaP), 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) and tert-butyl hydroperoxide (t-BOOH) in HepG2 human hepatoma cells. Mutat. Res., Genet. Toxicol. Environ. Mutagen 632, 1–8. [DOI] [PubMed] [Google Scholar]
- (63).Plazar J, Filipič M, and Groothuis GM (2008) Antigenotoxic effect of Xanthohumol in rat liver slices. Toxicol. In Vitro 22, 318–327. [DOI] [PubMed] [Google Scholar]
- (64).Viegas O, Zegura B, Pezdric M, Novak M, Ferreira IM, Pinho O, and Filipič M (2012) Protective effects of xanthohumol against the genotoxicity of heterocyclic aromatic amines MeIQx and PhIP in bacteria and in human hepatoma (HepG2) cells. Food Chem. Toxicol 50, 949–955. [DOI] [PubMed] [Google Scholar]
- (65).Ferk F, Huber WW, Filipič M, Bichler J, Haslinger E, Mišik M, Nersesyan A, Grasl-Kraupp B, Zegura B, and Knasmuller S (2010) Xanthohumol, a prenylated flavonoid contained in beer, prevents the induction of preneoplastic lesions and DNA damage in liver and colon induced by the heterocyclic aromatic amine amino-3-methyl-imidazo[4,5-f]quinoline (IQ). Mutat. Res. Fundam. Mol. Mech. Mutagen 691, 17–22. [DOI] [PubMed] [Google Scholar]
- (66).Pichler C, Ferk F, Al-Serori H, Huber W, Jäger W, Waldherr M, Mišik M, Kundi M, Nersesyan A, Herbacek I, and Knasmuller S (2017) Xanthohumol prevents DNA damage by dietary carcinogens: results of a human intervention trial. Cancer Prev. Res 10, 153–160. [DOI] [PubMed] [Google Scholar]
- (67).Bharti AC, and Aggarwal BB (2002) Nuclear factor-kappa B and cancer: its role in prevention and therapy. Biochem. Pharmacol 64, 883–888. [DOI] [PubMed] [Google Scholar]
- (68).Monteiro R, Calhau C, Silva AO, Pinheiro-Silva S, Guerreiro S, Gartner F, Azevedo I, and Soares R (2008) Xanthohumol inhibits inflammatory factor production and angiogenesis in breast cancer xenografts. J. Cell. Biochem 104, 1699–1707. [DOI] [PubMed] [Google Scholar]
- (69).Yadav VR, Prasad S, Sung B, and Aggarwal BB (2011) The role of chalcones in suppression of NF-kappaB-mediated inflammation and cancer. Int. Immunopharmacol 11, 295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Harikumar KB, Kunnumakkara AB, Ahn KS, Anand P, Krishnan S, Guha S, and Aggarwal BB (2009) Modification of the cysteine residues in IkappaBalpha kinase and NF-kappaB (p65) by xanthohumol leads to suppression of NF-kappaB-regulated gene products and potentiation of apoptosis in leukemia cells. Blood 113, 2003–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Jiang CH, Sun TL, Xiang DX, Wei SS, and Li WQ (2018) Anticancer activity and mechanism of xanthohumol: a prenylated flavonoid from hops (Humulus lupulus L.). Front. Pharmacol. 9, 530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Yang JY, Della-Fera MA, Rayalam S, and Baile CA (2007) Effect of xanthohumol and isoxanthohumol on 3T3-L1 cell apoptosis and adipogenesis. Apoptosis 12, 1953–1963. [DOI] [PubMed] [Google Scholar]
- (73).Strathmann J, Klimo K, Sauer SW, Okun JG, Prehn JH, and Gerhauser C (2010) Xanthohumol-induced transient superoxide anion radical formation triggers cancer cells into apoptosis via a mitochondria-mediated mechanism. FASEB J. 24, 2938–2950. [DOI] [PubMed] [Google Scholar]
- (74).Pan L, Becker H, and Gerhauser C (2005) Xanthohumol induces apoptosis in cultured 40–16 human colon cancer cells by activation of the death receptor- and mitochondrial pathway. Mol. Nutr. Food Res 49, 837–843. [DOI] [PubMed] [Google Scholar]
- (75).Guo D, Zhang B, Liu S, and Jin M (2018) Xanthohumol induces apoptosis via caspase activation, regulation of Bcl-2, and inhibition of PI3K/Akt/mTOR-kinase in human gastric cancer cells. Biomed. Pharmacother 106, 1300–1306. [DOI] [PubMed] [Google Scholar]
- (76).Sun Z, Zhou C, Liu F, Zhang W, Chen J, Pan Y, Ma L, Liu Q, Du Y, Yang J, and Wang Q (2017) Inhibition of breast cancer cell survival by Xanthohumol via modulation of the Notch signaling pathway in vivo and in vitro. Oncol. Lett 15, 908–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Dietz BM, and Bolton JL (2011) Biological reactive intermediates (BRIs) formed from botanical dietary supplements. Chem.-Biol Interact 192, 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Fernandez-Garcia C, Rancan L, Paredes SD, Montero C, de la Fuente M, Vara E, and Tresguerres JAF (2018) Xanthohumol exerts protective effects in liver alterations associated with aging. Eur. J. Nutr, DOI: 10.1007/s00394-018-1657-6. [DOI] [PubMed] [Google Scholar]
- (79).Dostalek P, Karabin M, and Jelinek L (2017) Hop Phytochemicals and Their Potential Role in Metabolic Syndrome Prevention and Therapy. Molecules 22, 1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Kirkwood JS, Legette LL, Miranda CL, Jiang Y, and Stevens JF (2013) A metabolomics-driven elucidation of the antiobesity mechanisms of xanthohumol. J. Biol. Chem 288, 19000–19013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Samuels JS, Shashidharamurthy R, and Rayalam S (2018) Novel anti-obesity effects of beer hops compound xanthohumol: role of AMPK signaling pathway. Nutr. Metab 15, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Takahashi K, and Osada K (2017) Effect of Dietary Purified Xanthohumol from Hop (Humulus lupulus L.) Pomace on Adipose Tissue Mass, Fasting Blood Glucose Level, and Lipid Metabolism in KK-Ay Mice. J. Oleo Sci 66, 531–541. [DOI] [PubMed] [Google Scholar]
- (83).Yui K, Kiyofuji A, and Osada K (2014) Effects of Xanthohumol-rich Extract from the Hop on Fatty Acid Metabolism in Rats Fed a High-fat Diet. J. Oleo Sci 63, 159–168. [DOI] [PubMed] [Google Scholar]
- (84).Cardaci S, Filomeni G, and Ciriolo MR (2012) Redox implications of AMPK-mediated signal transduction beyond energetic clues. J. Cell Sci 125, 2115–2125. [DOI] [PubMed] [Google Scholar]
- (85).Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, and Cantley LC (2004) The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. U. S. A 101, 3329–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Legette LL, Moreno Luna AY, Reed RL, Miranda CL, Bobe G, Proteau RR, and Stevens JF (2013) Xanthohumol lowers body weight and fasting plasma glucose in obese male Zucker fa/fa rats. Phytochemistry 91, 236–241. [DOI] [PubMed] [Google Scholar]
- (87).Gallo C, Dallaglio K, Bassani B, Rossi T, Rossello A, Noonan DM, D’Uva G, Bruno A, and Albini A (2016) Hop derived flavonoid xanthohumol inhibits endothelial cell functions via AMPK activation. Oncotarget 7, 59917–59931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Rudzitis-Auth J, Korbel C, Scheuer C, Menger MD, and Laschke MW (2012) Xanthohumol inhibits growth and vascularization of developing endometriotic lesions. Hum. Reprod 27, 1735–1744. [DOI] [PubMed] [Google Scholar]
- (89).Henderson MC, Miranda CL, Stevens JF, Deinzer ML, and Buhler DR (2000) In vitro inhibition of human P450 enzymes by prenylated flavonoids from hops, Humuîus îupuîus. Xenobiotica 30, 235–251. [DOI] [PubMed] [Google Scholar]
- (90).Yuan Y, Qiu X, Nikolic D, Chen SN, Huang K, Li G, Pauli GF, and van Breemen RB (2014) Inhibition of human cytochrome P450 enzymes by hops (Humulus lupulus) and hop prenylphenols. Eur. J. Pharm. Sci. 53, 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Dong J, Zhang Q, Cui Q, Huang G, Pan X, and Li S (2016) Flavonoids and naphthoflavonoids: wider roles in the modulation of cytochrome P450 family 1 enzymes. ChemMedChem 11, 2102–2118. [DOI] [PubMed] [Google Scholar]
- (92).Foster BC, Kearns N, Arnason JT, Saleem A, Ogrodowczyk C, and Desjardins S (2009) Comparative study of hop-containing products on human cytochrome p450-mediated metabolism. J. Agric. Food Chem 57, 5100–5105. [DOI] [PubMed] [Google Scholar]
- (93).Foster BC, Arnason JT, Saleem A, Tam TW, Liu R, Mao J, and Desjardins S (2011) Comparative study of hopscontaining products on human cytochrome P450-mediated metabolism. J. Agric. Food Chem 59, 5159–5163. [DOI] [PubMed] [Google Scholar]