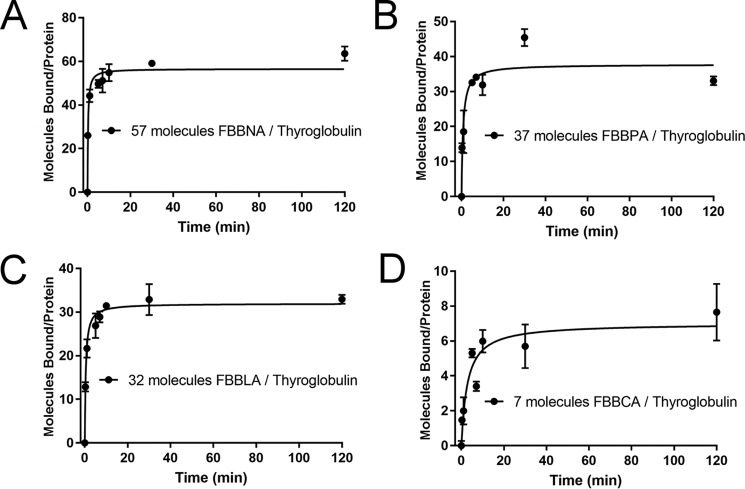
Figure 4.
Binding of candidate dye FBBNA compared with FBBPA, FBBLA, and FBBCA reveals the importance of the hydrophobic anchor region. As the hydrophobic portion of each coupling agent is reduced, the number of bound molecules of the corresponding dye is reduced. All data points were collected in duplicate and fit to the Michaelis–Menten equation, where Vmax represents maximum number of bound molecules at equilibrium. FBBNA had the highest number of molecules bound at equilibrium of the four candidate dyes tested. A, binding of FBBNA to thyroglobulin shows an average of 57 molecules bound to each thyroglobulin molecule at equilibrium, as determined previously and shown in Fig. 3A. The graph is reproduced in this figure for direct comparison with B, C, and D. B, binding of FBBPA to thyroglobulin shows an average of 37 molecules bound to each thyroglobulin molecule at equilibrium. C, binding of FBBLA to thyroglobulin shows an average of 32 molecules bound to each thyroglobulin molecule at equilibrium. D, binding of FBBCA to thyroglobulin shows an average of seven molecules bound to each thyroglobulin molecule at equilibrium. Error bars, S.D.