Abstract
Triple negative breast cancer (TNBC) is a highly aggressive cancer and lack of targeting therapies. It is believed that the breast cancer stem cells (BCSCs) are responsible for the aggressive characteristics of TNBC. Hence, developing BCSC-targeting agents may provide new therapeutic strategies for the patients. Huaier polysaccharide (HP), an active ingredient extracted from the mushroom Trametes robiniophila Murr, has been widely used in clinical anti-cancer treatments in China. Here we demonstrated that HP could target BCSCs in TNBC cells, resulting in decreased mammosphere formation, downregulated expression of stem-related genes and reduced proportion of aldehyde dehydrogenase positive cells in vitro, and inhibited xenograft tumor formation in vivo. Mechanically, HP markedly reduced the expression of estrogen receptor α-36 (ERα-36), a recently identified subtype of estrogen receptor α, and attenuated ERα-36-mediated activation of AKT/β-catenin signaling in ERα-36high TNBC cells. This study provides a new insight into the mechanism of HP on BCSC-targeting therapy and new ideas for comprehensive treatment strategies for TNBC.
Keywords: Huaier, ERα-36, Triple negative breast cancer, Cancer stem cells
Introduction
Breast cancer is an aggressive malignancy with the highest morbidity and mortality of all cancers in women 1. Triple negative breast cancer (TNBC) is a distinct subtype of breast cancer defined as negative for estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor-2 (Her2) and characterized by early onset of disease, low-grade cell differentiation, metastases, chemo-resistance causing recurrence, and lack of targeted therapies 2. It has been suggested that the cancer stem cells (CSCs) is responsible for the aggressive nature of TNBC 3-5. Therefore, developing new drugs targeting CSCs has become a promising therapeutic strategy for the patients with TNBC.
Many traditional Chinese herbal medicines (CHM) have shown their potential anti-cancer effects and applied in clinical practice for many years 6, 7. Trametes robiniophila murr (Huaier), an officinal fungus in China, is one of the anti-cancers CHM and has been used in different dosage forms for anti-cancer treatment of several cancers including breast cancer 8. Huaier polysaccharide (HP), an active ingredient extracted from the Trametes robiniophila Murr, is composed of 6 monosaccharides and 18 amino acids. Previous studies have demonstrated that administration of HP or Huaier aqueous extract significantly inhibits proliferation and promotes apoptosis in cancers of the liver 9, lung 10, ovarian11, and breast12. It has also been reported that HP or Huaier aqueous extract has an inhibitory effect on CSCs in MCF-7 breast cancer cell line and colorectal cancer cells by attenuating sHH and Wnt/β-catenin pathways, respectively 13, 14. However, the inhibitory effect of HP on TNBC and the underlying mechanisms need to be further clarified. In recent years, estrogen receptor α36 (ERα-36), a subtype of estrogen receptor α, has been demonstrated to be an important player in growth, self-renewal, differentiation and tumor seeding of breast cancer stem cells (BCSCs) 15-17. So we hypothesized that HP may attenuate ERα-36 signaling to inhibit BCSCs in TNBC.
In this study, we demonstrated that HP can effectively reduce CSC compartment by attenuating ERα-36-mediated activation of AKT/β-catenin signaling in ERα-36high TNBC cells and provided new ideas for comprehensive treatment for ERα-36high subtype of TNBC.
Results
HP inhibits proliferation and mammosphere formation in TNBC cells
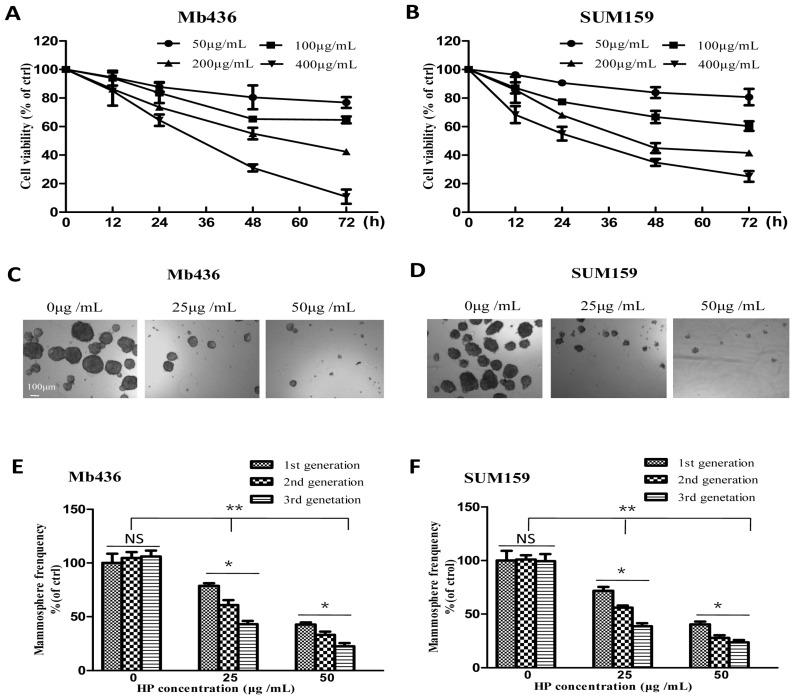
First, the cytotoxic effects of HP (The purity is over 99%, Figure S1) on the TNBC cell lines Mb436 and SUM159 were evaluated. As shown in Figure 1A and B, the viability of HP-treated Mb436 and SUM159 cells was decreased in a time- and dose-dependent manner. The half inhibitory concentrations (IC50) of HP for Mb436 and SUM159 cells were 205.12 ± 36.41 and 195.34 ± 27.62 μg/mL at 48 h, respectively.
Figure 1.
HP inhibits TNBC cell viability and mammosphere growth. (A-B) Cytotoxicity of different concentrations of HP on Mb436 and SUM159 cells detected by CCK-8 kit at 12, 24, 48 and 72h. (C-D) Representative images showed that first generation mammospheres of Mb436 and SUM159 cells were significantly reduced after treatment with different concentrations of HP for 7 days (40×; scale bar, 100μm). (E-F) Quantitation (normalized to respective control) of first, second and third generation mammospheres of Mb436 and SUM159 cells after treatment with different concentrations of HP for 7 days. Data are presented as means ± SD (n= 3).*, P<0.05 and **, P<0.01.
Given the enrichment of CSCs and progenitor cells under non-adherent and serum-free culture conditions 18, a mammosphere formation assay was used to evaluate the inhibitory effects of HP on the stemness characteristics in TNBC cells. As shown in Figure 1C and D, HP treatment markedly reduced the quantity and size of mammospheres in a dose-dependent manner in Mb436 and SUM159 cells. Moreover, mammosphere-forming capability of the second and third generations was also significantly lower in HP-treated cells, as compared to the controls (Figure 1E and F). These results suggest that HP can inhibit the self-renewal capability of BCSCs in TNBC.
HP effectively down-regulates expression of stem-related genes and reduces ALDH1+ population in TNBC cells
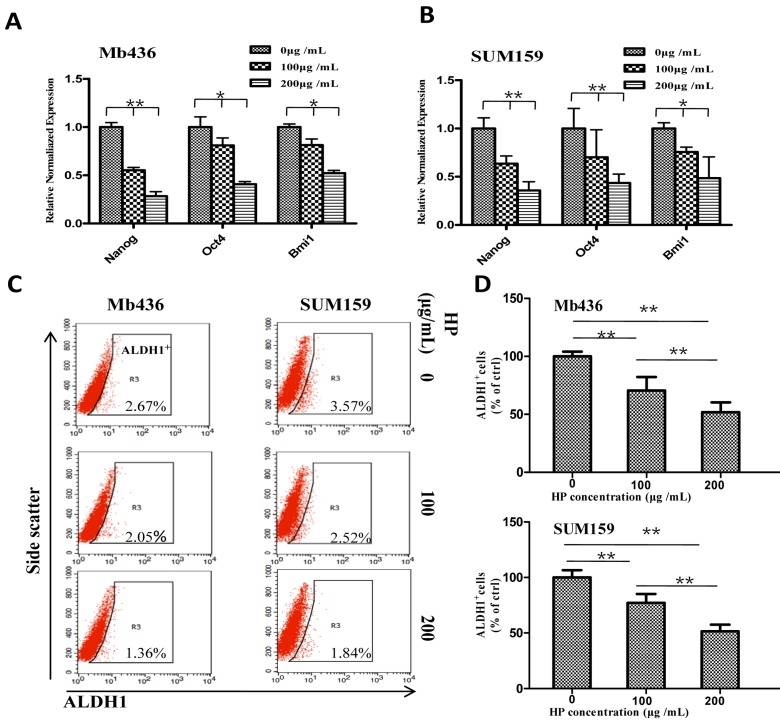
We further evaluated the influence of HP on the stemness of TNBC cells by comparing the expression of stem-related transcription factors Nanog, Oct4 and Bmi1in TNBC cells treated with or without HP. HP treatment significantly decreased mRNA levels of the transcription factors in a dose-dependent manner in Mb436 (Figure 2A) and SUM159 (Figure 2B) cells (P<0.05 for both). It is well known that ALDH1+ cells are a subpopulation of BCSCs 19. Therefore, the effect of HP on ALDH1+ population was examined, and the results showed that HP treatment significantly reduced ALDH1+ population in a dose-dependent manner with a reduction of about 30% at 100 μg/mL, 72 h and 50% at 200 μg/mL, 72 h (p<0.05 for all) in both Mb436 and SUM159 cells (Figure 2C and D). These results showed that HP is an effective agent to reduce BCSC subpopulation in TNBC cells.
Figure 2.
HP downregulates the expression of stem-related genes and reduces ALDH1+ subpopulation in TNBC cells. (A-B) qRT-PCR showed that HP treatment downregulated the expression of stem-related genes Nanog, Oct4, and Bmi1 in Mb436 and SUM159 cells, compared to control (0 μg/mL). (C) Representative images showed that HP treatment (48 h) reduced ALDH1+ subpopulations in Mb436 and SUM159 cells. (D) Statistical histogram of HP reducing ALDH1+ subpopulationin Mb436 and SUM159 cells, compared to the control (0 μg/mL). Data are presented as means ± SD (n= 3). *, P<0.05 and **, P<0.01.
The inhibitory effect of HP on the stemness of TNBC cells is associated with ERα-36
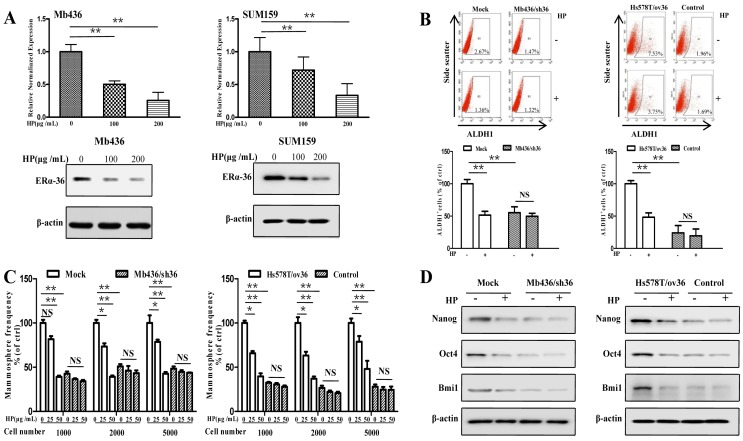
Recent studies have shown that ERα-36-mediated rapid estrogen signaling positively regulates BCSCs/progenitor cells 20. A question was raised as to whether the inhibitory effect of HP on stem-like characteristics of TNBC cells was associated with ERα-36. To address this issue, we first examined the expression of ERα-36 in TNBC cell lines and found that the expression levels were varied in different TNBC cells, with Mb436 and SUM159 cells highest, Mb231 and Mb453 cells medium and Hs578T cells lowest (Figure S2). Treatment with HP effectively inhibited the expression of ERα-36 in Mb436 and SUM159 cells (Figure 3A). We selected Mb436 and Hs578T cells to establish ERα-36-knockdown and -overexpression cell models, respectively (Figure S3). Silencing ERα-36 expression or treatment with HP resulted in about 50% reduction of ALDH1 positive cells in Mb436 mock cells, but hardly altered the proportion in Mb436/sh36 cells (Figure 3B, left panel). Both treatment with HP and ERα-36-knockdown markedly decreased the mammosphere formation in Mb436 cells, while treatment with HP hardly affected the mammosphere formation in Mb436/Sh36 cells (Figure 3C, left panel). As shown in Figure 3D left panel, the expression of stem-related genes, Nanog, Oct4 and Bmi1, in Mb436 Mock cells were dramatically interrupted by ERα-36 knockdown or by HP treatment, while no effect of HP treatment on those genes was observed in Mb436/sh36 cells. However, the proportion of ALDH1 positive cells, the ability of mammosphere formation and the expression level of the stem-related genes were less affected by HP treatment in low ERα-36-expression Hs578T cells (control cells), but the significantly inhibitory effect was observed upon HP treatment in ERα-36-overexpression Hs578T (Hs578T/ov36) cells, which exhibited enhanced stem properties (Figure 3B-D, right panels). These results indicate that ERα-36 is involved in the inhibitory effect of HP on the stemness of TNBC cells.
Figure 3.
ERα-36 signaling is involved in the inhibitory effect of HP on stem-like characteristics of TNBC cells in vitro. (A) qRT-PCR and western blot showed that HP treatment downregulated the expression of ERα-36 in Mb436 and SUM159 cells. (B) ALDH1+ subpopulation was reduced by treatment with HP (200 μg/mL) for 48h in Mb436 Mock and Hs578T/sh36 cells, but not in Mb436/sh36 and Hs578T cells analyzed by Flow cytometry. The statistical histogram showed the percentage of ALDH1+ cells relative to control cells (0 μg/mL). (C) In Mb436 Mock and Hs578T/ov36 cells, the frequency of mammosphere formation was markedly inhibited by treatment with different doses of HP for 7 days, while in ERα-36-knockdown Mb436/sh36 and Hs578Tcells, HP treatment hardly affected the frequency of mammosphere formation. (D) Western blot analyses showed that HP treatment downregulated the expression of Nanog, Oct4, and Bmi1 in Mb436 Mock and Hs578T/ov36 cells, but not in Mb436/sh36 and Hs578T cells. (*, P<0.05, **, P<0.01 and NS, no significance).
HP inactivates ERα-36-mediated AKT/GSK3β/β-catenin pathway in TNBC cells
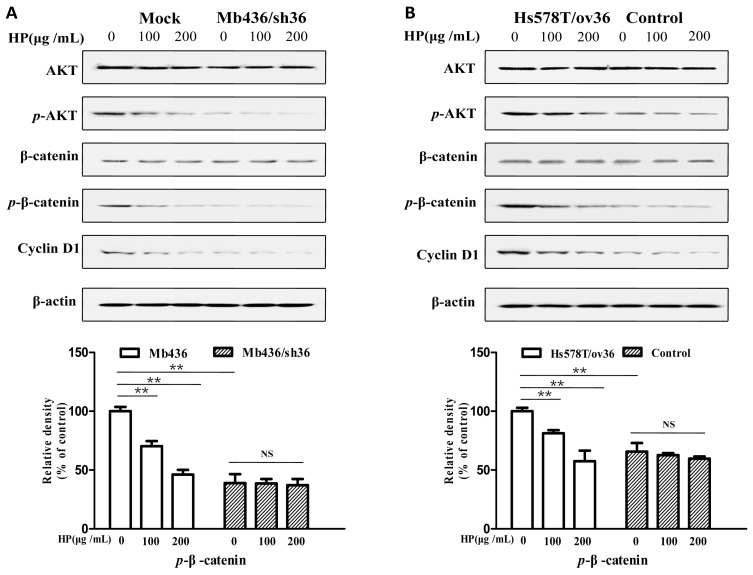
It has been reported that the AKT/GSK3β/β-catenin pathway is involved in ERα-36-mediated estrogen signaling in BCSCs/progenitor cells 16, 20. Therefore, we investigated whether the effect of HP on TNBC cells associated with ERα-36-mediated estrogen signaling. As shown in Figure 4A, Mb436 mock cells had higher AKT phosphorylation and consequently high expression levels of p-β-catenin and cyclin D1, a downstream gene of β-catenin signaling, compared to Mb436/sh36 cells. Treatment with HP in Mb436 Mock cells resulted in a dramatically decreased phosphorylation of AKT and interrupted expression of p-β-catenin, and cyclin D1. However, HP treatment could not alter the expression levels of p-AKT, p-β-catenin and cyclin D1 in Mb436/sh36 cells. As shown in Figure 4B, the inhibitory effect of HP on the expression levels of p-AKT, p-β-catenin, and cyclin D1 became evident after over-expressing ERα-36 in Hs578T cells, as compared to control cells. These results strongly support that inhibitory effect of HP on TNBC cells is closely associated with ERα-36 pathway.
Figure 4.
HP deactivates ERα-36-mediated AKT/GSK3β/β-catenin signaling pathway in TNBC cells HP treatment reduced the p-AKT and downregulated the expression of p-β-catenin and cyclin D1, the downstream genes of ERα-36 signaling, in Mb436 Mock cells (Figure 4A) and Hs578T/ov36 cells (Figure 4B), but not in Mb436/sh36 and Hs578T cells. Data are presented as means ± SD (n= 3). (**, P<0.01 and NS, no significance).
Silencing ERα-36 expression overwhelms the inhibitory effect of HP on tumor formation of TNBC cells in vivo
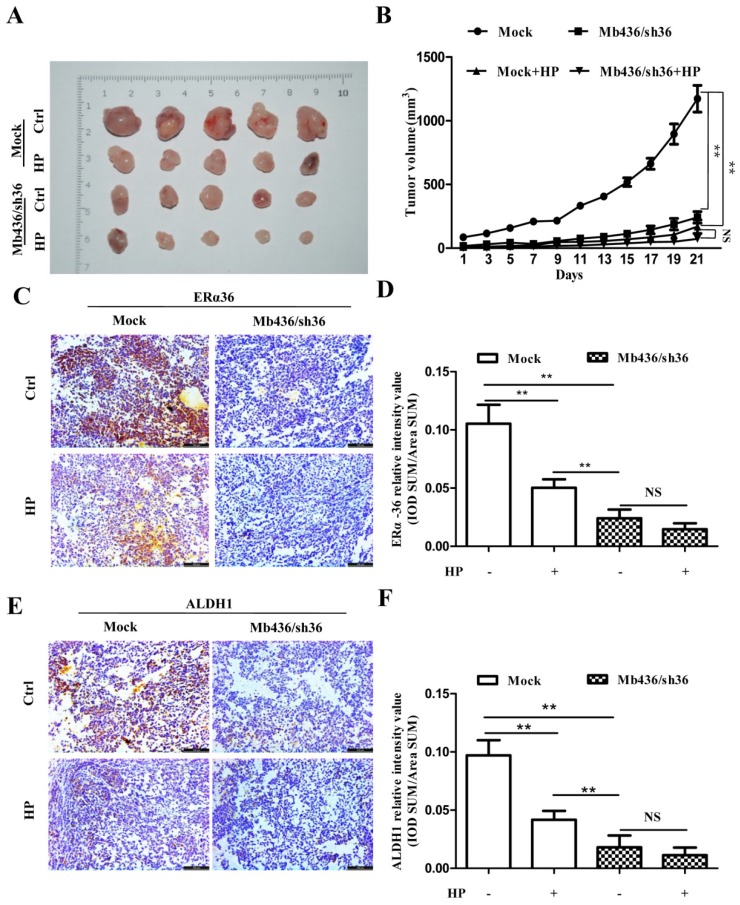
To assess the efficacy of HP on TNBC cells in vivo, a NOD/SCID mouse orthotopic xenograft model was used. Mb436/Sh36 and mock cells were injected into the left and right breast pads of NOD/SCID mice, respectively. When the tumor size grew to about 40 mm3, the mice were randomly allocated to either the control group or the experimental group (n=5). The mice of experimental group were subjected to intragastric administration of HP, 60mg/kg, once a day for 3weeks. The same treatment is applied to the mice of control group, replacing HP with normal saline solution. In tumor-bearing mice implanted with Mock cells, the size of xenograft tumors in experimental group was markedly smaller than that of control group (P<0.01) (Figure 5A and B). Compared to mock cell group, silencing ERα-36 significantly impaired the growth of xenograft tumors (P<0.01), while HP treatment showed relatively smaller effect on the tumors derived from Mb436/sh36 (P>0.05) (Figure 5 A and B).
Figure 5.
ERα-36 knockdown overwhelms the inhibitory effect of HP on xenograft tumors of TNBC cells. (A) Images of xenograft tumors showed that HP treatment significantly decreased the size of tumors in Mb436 Mock cell-derived tumor group, compared to control. Compared with Mb436 Mock cell-derived tumor group, the sizes of Mb436/sh36 cell-derived tumors were markedly reduced and HP treatment could not further reduce the sizes of tumors. (B) Statistics of tumor sizes in different groups. (C) IHC images showed that HP treatment significantly decreased the expression of ERα-36 in Mb436 Mock cell-derived tumor group, compared to control. (D) Statistics of IHC scores for ERα-36 in different groups. (E) IHC images showed that compared to control, HP treatment significantly decreased the expression of ALDH1 in Mb436 Mock cell-derived tumor group, but not in Mb436/sh36 cell-derived tumor group. (F) Statistics of IHC scores for ALDH1 in different groups. Data are presented as means ± SD (n= 3). (**, p <0.01 and NS, no significance).
To confirm that HP inhibited the formation and growth of xenograft tumors by attenuating ERα-36, the expression of ERα-36 in xenograft tumors treated with or without HP was measured by immunohistochemical staining. HP treatment reduced the ERα-36 expression in Mb436 mock cell-derived xenograft tumors, compared to control (P<0.01) (Figure 5C and D). HP treatment also significantly decreased the expression of ALDH1 in the Mb436 mock cell-derived xenograft tumors (P<0.01), while this effect was overwhelmed by ERα-36 knockdown (P>0.05) (Figure 5E and F). These results indicate that ERα-36 signaling is involved in the effect of HP on inhibiting the growth of TNBC xenograft tumors.
Discussion
Identifying specific targets and developing more effective, dynamic and promising therapies for TNBC patients has been an important clinical challenge. In recent years, though a number of regimens with single or comprehensive agents have been developed for the treatment of TNBC, few of them is specifically designed for this disease and the clinical results have been somewhat disappointing 21. In general, the sensitivity of TNBC to chemotherapy is high, whereas the overall outcome is poor. The paradox may be attributed in part to the presence of BCSCs within TNBC tumors, which are defined as CD44+/CD24- or ALDH1+ cell population 19, 22. It has been demonstrated that BCSCs not only possess the ability to self-renew, invade and metastasize, but also resist to conventional chemotherapy to become residual cancer cells after treatment 23-25. Clinical studies showed that neoadjuvant chemotherapy results in the enrichment of BCSCs in human patients with breast cancer 26, 27. Moreover, Lin et al. reported that increased percent of BCSCs significantly associates with poorer prognosis of breast cancer patients 28. Therefore, targeting BCSCs in TNBC may be a promising therapeutic strategy for TNBC patients.
A straight forward approach to targeting BCSCs may be the use of natural compounds often found in dietary sources and Chinese herbs. In recent years, a number of them, such as curcumin, resveratrol and so on, have been found to have a role in targeting BCSCs 29. It has also been reported that Huaier aqueous extract inhibited stem-like characteristics of MCF7 cells 13. In this study, we further demonstrated that HP reduced the mamosphere formation, stem-related gene expression, the subpopulation of ALDH1+ and inhibited the growth of xenograft tumors in TNBC cells, suggesting that the active ingredient of Huaier is an effective agent for targeting BCSCs in TNBC.
The anti-cancer effect of Huaier extract involved multiple signaling pathways. For example, Yan et al. reported that Huaier inhibited tumor cell mobility in ovarian cancer via the AKT/GSK3β/β-catenin pathway 11. Zhang et al. demonstrated that Huaier aqueous extract targeted colorectal CSCs by inhibiting Wnt/β-catenin pathway 14. Wang et al. also reported that Huaier extract inhibited BCSCs partially through inhibiting sHH signaling pathway 13. These results suggest that the inhibitory effect of HP on BCSCs in TNBC may involve multiple signaling pathways.
Breast cancer is a hormone-related disease. Estrogen receptorα (ERα) plays a critical role in the growth of breast cancer cells, and associates with patient's prognosis as well as endocrine therapy 30, 31. Recent studies have reported that ERα-36, a variant of ERα-66, is highly expressed in ERα-66 negative tumor tissues and cell lines 32, 33, and plays an important role in the carcinogenesis and progression of cancer through involving in tumor cell proliferation, differentiation and metastasis 15, 34, 35. Moreover, Wang et al. also demonstrated that up-regulating ERα-36 expression can stimulate the self-renewal of CSCs and expand the population of CSCs in breast cancer cells 20. In present study, we demonstrated that the inhibitory effect of HP on stem-like characteristics of TNBC BCSCs is at least partially associated with ER-α36. Estrogenic effects could be induced by genomic and nongenomic pathways 36. Recent studies have reported that ERα-36 is localized in the cell membrane and cytoplasm, where it mainly participates in the initiation of nongenomic pathways to activate PI3K-Akt and MAPK/ERK signaling pathways 37. A previous study indicated that ERα-36-mediated rapid estrogen signaling can positively regulate the proliferation of breast CSCs/progenitor cells 20. The present study further demonstrated that HP inhibited the expression of p-Akt, p-β-catenin, and cyclin D1 in a dose-dependent manner. However, how HP affects the expression of ERα-36 needs further studies.
In conclusion, the findings of this study demonstrated that HP inhibited the stem-like characteristics of TNBC cells both in vitro and in vivo partly through the ERα-36 signaling pathway. These findings support the use of HP as an effective supplementary treatment for TNBC.
Materials and Methods
Cell culture
All the cells used in this study were all purchased from the American Type Culture Collection. MDA-MB-436 (Mb436) cells were maintained in L15 (HyClone, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, USA). SUM159 cells were maintained in Ham's F12 medium (Invitrogen, USA) supplemented with 5% FBS, 5 μg/mL insulin, and 1 μg/mL hydrocortisone (Santa Cruz, USA). MDA-MB-231 (Mb231),MDA-MB-453(Mb453) and Hs578T cells were maintained in DMEM containing 10% fetal bovine serum (FBS). All cells were cultured at 37°C in 5% CO2 and 100% humidity.
Establishment of stable ERα-36-knockdown and -overexpression breast cancer cells
Lentivirus particles containing ERα-36-specific shRNAs and a scramble shRNA were obtained from Life Technologies Co. Ltd (Shanghai, China). The sequences targeting ERα-36 and the non-targeting sequence were listed in Table S1. After infecting Mb436 cells with 2 μg/mL of polybrene 17, ERα-36-stable-knockdown cells were selected using FACS. The ERα-36-knockdown cells were named as Mb436/sh36, whereas the control cells were named as Mock. The cells of ERα-36-overexpression were stably established as previously described 17. The ERα-36-overexpressing cells were named as Hs578T/ov36, whereas the vector-transfected cells were named as control.
HP isolation and purification
HP crude extract was obtained from Qidong Gaitianli Pharmaceutical Co., Ltd. (Qidong, China). HP was isolated and purifiedas previously described 38. Briefly, crude HP was obtained by dehydration and distillation of the fruiting bodies, and applied to a diethylaminoethyl (DEAE) cellulose-52 (Amersham Pharmacia, Sweden) chromatography column; then it was washed with distilled water and eluted with 0.1~0.3 mol/L sodium chloride. The purity of HP was evaluated by a phenol-sulfuric acid method with glucose as the standard 39 .
Cell proliferation assay
Mb436 and SUM159 cells were seeded in 96-well microplates at a density of 3000 cells/well and pre-incubated for 24 h. Then different concentrations of HP were added and the absorbance at 450 nm was determined at 12, 24, 48 and 72 h with CCK-8 kit (TaKaRa, Japan) on a microplate reader.
Sphere formation assay
Cells were seeded in Ultra-Low Attachment plates (Corning, USA) at a concentration of 1000-5000cells/mL in DMEM/F12 serum-free medium containing 10 ng/mL basic fibroblast growth factor (Invitrogen, USA), 10ng/mL epidermal growth factor (Sigma-Aldrich, USA), 0.5 μg/mL hydrocortisone (Sigma-Aldrich, USA)and 1×B27cell culture supplement (Invitrogen, USA). Indicated concentrations of HP were added to the media and cultured for seven days. Then the size and number of mammospheres were observed and calculated. For the generation of mammosphere cells, the primary mammospheres were harvested and dissociated with trypsin. After passing through a 40 μm-pore strainer, the single cells were re-plated in ultra-low attachment plates at absence of HP to form the second and third generation mammospheres (7 days each).
Flow cytometry analysis
The analysis and sorting of ALDH1+ cells were performed using an AldefluorTM assay kit (STEMCELL Technologies,Canada) in accordance with the manufacturer's guidelines. Briefly, the cells were suspended in buffer containing ALDH1 substrate (Bodipy™-aminoacetaldehyde) and incubated at 37°C for 30min. As a control, half of the cells were treated with the ALDH1 inhibitor N,N-diethylaminobenzaldehyde, washed twice with buffer, re-suspended, and then analyzed with a fluorescence-activated cell sorting instrument (BD FACS Aria II).
Real-time quantitative reverse transcription ploymerase chain reaction (qRT-PCR)
Total RNA was extracted with TRIzolTM reagent (TaKaRa, Japen) and cDNA was synthesized using Prime Script RT reagent kit (TaKaRa) following the manufacturer's instructions. All the primers used in the experiments were available in Table S2.All reactions were performed in triplication using the TaqMan® Gene Expression Assay (Applied Biosystems, USA) and Universal PCR Master Mix. The expression level of each gene relative to GAPDH was calculated according to the 2-∆∆CT method. All experiments were repeated at least three times.
Western blot analysis
Cells were washed with cold phosphate-buffered saline (PBS) and then lysed with lysis buffer(Sigma-Aldrich Corporation) following the manufacturer's instructions. Protein concentration was measured using a BCA protein assay kit (Beyotime, China). Identical amount of proteins from different-treated samples was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred to polyvinylidene difluoride membranes (Millipore,USA). After blocking with PBST-5% skimmed milk, the membranes were incubated with indicated specific primary antibodies, including anti-ERα-36 antibody (generated and characterized as described before17), anti-ALDH1 (Abcam, UK), anti-AKT(Santa Cruz, USA), anti-pAKT (Santa Cruz, USA), anti-β-catenin (Santa Cruz, USA), anti-p-β-catenin (Santa Cruz, USA), anti-Bmi1 (Abcam, UK), anti-Nanog (Abcam, UK) and anti-Oct4 (Abcam, UK). After incubation overnight at 4°C, the membranes were washed and incubated with HRP-conjugated secondary antibodies. Finally, the membranes were visualized using enhanced chemiluminescence detection reagents (GE, USA).
Orthotopic xenograft tumorassay
Non-obese diabetic/severe combined immunodeficiency (NOD/SCID) mice, aged 4-6 weeks, were purchased from the Beijing Experimental Center (Beijing, China) and housed in clean laboratory with ad libitum access to food and water. One million Mb436/Sh36 cells and Mock cells were suspended in 60μL of L15/matrigel (1:1) (BD Biosciences, USA), respectively. The cells then were respectively injected into the left and right breast pads of the mice. When the tumor grew to about 40 mm3, the mice were randomly assigned to control group or experimental group (n=5). The experimental group received intragastric administration of 60 mg of HP/kg body weight once daily for 3 weeks, while the control group was given normal saline solution by gavage. At the end of treatment, all mice were euthanized to harvest the tumors. Tumor volume was calculated as (tumor length × width2) /2 40.
Immunohistochemical staining
Xenograft tumors were prepared into 5μm-thick paraffin sections. Then, the slides were deparaffinized, rehydrated, antigen retrieval and blocking followed by incubated with primary antibodies anti-ERα-36 antibody (1:200 dilution) and anti-ALDH1 antibody (1:100 dilution) overnight at 4°C. After washing with PBS, a secondary antibody was added and incubated at room temperature for 1 h, and then stained with 3,3'-diaminobenzidine (DAKO, Denmark). IHC scoring was performed as previously reported 41.
Statistical analysis
Statistical analyses were performed using SPSS 19.0 software (SPSS, USA). All quantitative parameters were presented as the mean ± standard deviation (SD). Two-tailed Student's t-tests were used for comparison between two groups using SPSS 19.0 software (SPSS, USA), and P< 0.05 was considered statistically significant.
Supplementary Material
Supplementary figures and tables.
Acknowledgments
This work was supported by Xiao-ping Chen Science and Development Foundation (No. CXPJJH1700001) and the National Natural Science Foundation of China (No. 81602730).
Author Contributions
J.J. and Q.X.W. conceived of the project and contributed to study design; Y.W.T. and H.Y. developed the methodology; H.B.Q., C.X., W.M.H and C.Q.Q. performed experiments, data collection and analysis; H.B.Q, Q.X.W and Z.Y. wrote and revised the manuscript; all authors read and approved the final version of the manuscript.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Ismail-Khan R, Bui MM. A review of triple-negative breast cancer. Cancer control: journal of the Moffitt Cancer Center. 2010;17:173–6. doi: 10.1177/107327481001700305. [DOI] [PubMed] [Google Scholar]
- 3.Margaryan NV, Seftor EA, Seftor REB, Hendrix MJC. Targeting the Stem Cell Properties of Adult Breast Cancer Cells: Using Combinatorial Strategies to Overcome Drug Resistance. Current molecular biology reports. 2017;3:159–64. doi: 10.1007/s40610-017-0067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rangel MC, Bertolette D, Castro NP, Klauzinska M, Cuttitta F, Salomon DS. Developmental signaling pathways regulating mammary stem cells and contributing to the etiology of triple-negative breast cancer. Breast cancer research and treatment. 2016;156:211–26. doi: 10.1007/s10549-016-3746-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stratford AL, Reipas K, Maxwell C, Dunn SE. Targeting tumour-initiating cells to improve the cure rates for triple-negative breast cancer. Expert reviews in molecular medicine. 2010;12:e22. doi: 10.1017/S1462399410001535. [DOI] [PubMed] [Google Scholar]
- 6.The Lancet O. Rethinking traditional Chinese medicines for cancer. The Lancet Oncology. 2015;16:1439. doi: 10.1016/S1470-2045(15)00406-4. [DOI] [PubMed] [Google Scholar]
- 7.Wang CY, Bai XY, Wang CH. Traditional Chinese medicine: a treasured natural resource of anticancer drug research and development. The American journal of Chinese medicine. 2014;42:543–59. doi: 10.1142/S0192415X14500359. [DOI] [PubMed] [Google Scholar]
- 8.Song X, Li Y, Zhang H, Yang Q. The anticancer effect of Huaier (Review) Oncology reports. 2015;34:12–21. doi: 10.3892/or.2015.3950. [DOI] [PubMed] [Google Scholar]
- 9.Zheng J, Li C, Wu X, Liu M, Sun X, Yang Y. et al. Huaier polysaccharides suppresses hepatocarcinoma MHCC97-H cell metastasis via inactivation of EMT and AEG-1 pathway. International journal of biological macromolecules. 2014;64:106–10. doi: 10.1016/j.ijbiomac.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 10.Wu T, Chen W, Liu S, Lu H, Wang H, Kong D. et al. Huaier suppresses proliferation and induces apoptosis in human pulmonary cancer cells via upregulation of miR-26b-5p. FEBS letters. 2014;588:2107–14. doi: 10.1016/j.febslet.2014.04.044. [DOI] [PubMed] [Google Scholar]
- 11.Yan X, Lyu T, Jia N, Yu Y, Hua K, Feng W. Huaier aqueous extract inhibits ovarian cancer cell motility via the AKT/GSK3beta/beta-catenin pathway. PloS one. 2013;8:e63731. doi: 10.1371/journal.pone.0063731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Zhang N, Huo Q, Sun M, Lv S, Yang Q. Huaier aqueous extract suppresses human breast cancer cell proliferation through inhibition of estrogen receptor alpha signaling. International journal of oncology. 2013;43:321–8. doi: 10.3892/ijo.2013.1947. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Zhang N, Huo Q, Sun M, Dong L, Zhang Y. et al. Huaier aqueous extract inhibits stem-like characteristics of MCF7 breast cancer cells via inactivation of hedgehog pathway. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2014;35:10805–13. doi: 10.1007/s13277-014-2390-2. [DOI] [PubMed] [Google Scholar]
- 14.Zhang T, Wang K, Zhang J, Wang X, Chen Z, Ni C. et al. Huaier aqueous extract inhibits colorectal cancer stem cell growth partially via downregulation of the Wnt/beta-catenin pathway. Oncology letters. 2013;5:1171–6. doi: 10.3892/ol.2013.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang ZY, Yin L. Estrogen receptor alpha-36 (ER-alpha36): A new player in human breast cancer. Molecular and cellular endocrinology. 2015;418(Pt 3):193–206. doi: 10.1016/j.mce.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 16.Deng H, Yin L, Zhang XT, Liu LJ, Wang ML, Wang ZY. ER-alpha variant ER-alpha36 mediates antiestrogen resistance in ER-positive breast cancer stem/progenitor cells. The Journal of steroid biochemistry and molecular biology. 2014;144( Pt B):417–26. doi: 10.1016/j.jsbmb.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 17.Wang Q, Jiang J, Ying G, Xie XQ, Zhang X, Xu W. et al. Tamoxifen enhances stemness and promotes metastasis of ERalpha36(+) breast cancer by upregulating ALDH1A1 in cancer cells. Cell research. 2018;28:336–58. doi: 10.1038/cr.2018.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ. et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes & development. 2003;17:1253–70. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M. et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell stem cell. 2007;1:555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng H, Zhang XT, Wang ML, Zheng HY, Liu LJ, Wang ZY. ER-alpha36-mediated rapid estrogen signaling positively regulates ER-positive breast cancer stem/progenitor cells. PloS one. 2014;9:e88034. doi: 10.1371/journal.pone.0088034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Cao S, Chen Y. Molecular Treatment of Different Breast Cancers. Anti-cancer agents in medicinal chemistry. 2015;15:701–20. doi: 10.2174/1871520615666150129211901. [DOI] [PubMed] [Google Scholar]
- 22.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheridan C, Kishimoto H, Fuchs RK, Mehrotra S, Bhat-Nakshatri P, Turner CH. et al. CD44+/CD24- breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast cancer research: BCR. 2006;8:R59. doi: 10.1186/bcr1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P. et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer research. 2009;69:1302–13. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF. et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. Journal of the National Cancer Institute. 2008;100:672–9. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 26.Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A. et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13820–5. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanei T, Morimoto K, Shimazu K, Kim SJ, Tanji Y, Taguchi T. et al. Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential Paclitaxel and epirubicin-based chemotherapy for breast cancers. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15:4234–41. doi: 10.1158/1078-0432.CCR-08-1479. [DOI] [PubMed] [Google Scholar]
- 28.Lin Y, Zhong Y, Guan H, Zhang X, Sun Q. CD44+/CD24- phenotype contributes to malignant relapse following surgical resection and chemotherapy in patients with invasive ductal carcinoma. Journal of experimental & clinical cancer research: CR. 2012;31:59. doi: 10.1186/1756-9966-31-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dandawate PR, Subramaniam D, Jensen RA, Anant S. Targeting cancer stem cells and signaling pathways by phytochemicals: Novel approach for breast cancer therapy. Seminars in cancer biology. 2016;40-41:192–208. doi: 10.1016/j.semcancer.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fisher B, Redmond C, Fisher ER, Caplan R. Relative worth of estrogen or progesterone receptor and pathologic characteristics of differentiation as indicators of prognosis in node negative breast cancer patients: findings from National Surgical Adjuvant Breast and Bowel Project Protocol B-06. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1988;6:1076–87. doi: 10.1200/JCO.1988.6.7.1076. [DOI] [PubMed] [Google Scholar]
- 31.Kinne DW, Butler JA, Kimmel M, Flehinger BJ, Menendez-Botet C, Schwartz M. Estrogen receptor protein of breast cancer in patients with positive nodes. High recurrence rates in the postmenopausal estrogen receptor-negative group. Archives of surgery (Chicago, Ill: 1960) 1987;122:1303–6. doi: 10.1001/archsurg.1987.01400230089016. [DOI] [PubMed] [Google Scholar]
- 32.Shi L, Dong B, Li Z, Lu Y, Ouyang T, Li J. et al. Expression of ER-{alpha}36, a novel variant of estrogen receptor {alpha}, and resistance to tamoxifen treatment in breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27:3423–9. doi: 10.1200/JCO.2008.17.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. A variant of estrogen receptor-{alpha}, hER-{alpha}36: transduction of estrogen- and antiestrogen-dependent membrane-initiated mitogenic signaling. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9063–8. doi: 10.1073/pnas.0603339103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaudhri RA, Hadadi A, Lobachev KS, Schwartz Z, Boyan BD. Estrogen receptor-alpha 36 mediates the anti-apoptotic effect of estradiol in triple negative breast cancer cells via a membrane-associated mechanism. Biochimica et biophysica acta. 2014;1843:2796–806. doi: 10.1016/j.bbamcr.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaudhri RA, Olivares-Navarrete R, Cuenca N, Hadadi A, Boyan BD, Schwartz Z. Membrane estrogen signaling enhances tumorigenesis and metastatic potential of breast cancer cells via estrogen receptor-alpha36 (ERalpha36) The Journal of biological chemistry. 2012;287:7169–81. doi: 10.1074/jbc.M111.292946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. Identification, cloning, and expression of human estrogen receptor-alpha36, a novel variant of human estrogen receptor-alpha66. Biochemical and biophysical research communications. 2005;336:1023–7. doi: 10.1016/j.bbrc.2005.08.226. [DOI] [PubMed] [Google Scholar]
- 37.Lin SL, Yan LY, Zhang XT, Yuan J, Li M, Qiao J. et al. ER-alpha36, a variant of ER-alpha, promotes tamoxifen agonist action in endometrial cancer cells via the MAPK/ERK and PI3K/Akt pathways. PloS one. 2010;5:e9013. doi: 10.1371/journal.pone.0009013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun Y, Sun T, Wang F, Zhang J, Li C, Chen X. et al. A polysaccharide from the fungi of Huaier exhibits anti-tumor potential and immunomodulatory effects. Carbohydrate polymers. 2013;92:577–82. doi: 10.1016/j.carbpol.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 39.Dubois M, Gilles K, Hamilton JK, Rebers PA, Smith F. A colorimetric method for the determination of sugars. Nature. 1951;168:167. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- 40.Yang L, Ping YF, Yu X, Qian F, Guo ZJ, Qian C. et al. Gastric cancer stem-like cells possess higher capability of invasion and metastasis in association with a mesenchymal transition phenotype. Cancer letters. 2011;310:46–52. doi: 10.1016/j.canlet.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Ji CD, Wang YX, Xiang DF, Liu Q, Zhou ZH, Qian F. et al. Kir2.1 interaction with Stk38 promotes invasion and metastasis of human gastric cancer by enhancing MEKK2-MEK1/2-ERK1/2 signaling. Cancer research. 2018;78:3041–53. doi: 10.1158/0008-5472.CAN-17-3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures and tables.