Abstract
Background
Dentin hypersensitivity is a common negative oral condition that can be treated with dentifrice containing hydroxyapatite (HA). The study evaluated the effect of nano-HA dentifrice on plugging the dentinal tubules for an anti-sensitivity reaction compared to a dentifrice containing common-sized particles. Also, the adsorption capacity of different particle sizes of HA mixed in a dentifrice and which is the optimal particle size was considered.
Methods
Fourty premolar dentine discs and fourty molar dentine discs were randomly divided into 4 groups: distilled water group, ordinary dentifrice group and 80, 300 nm HA dentifrice group. Each dentin disc was brushed with a dentifrice twice daily at 7600 rpm under 100 g force for 2 mins for 7 consecutive days and divided into two parts, half of the dentin disc was detected by the scanning electron microscope (SEM) and energy dispersive spectrometer (EDS), the other half was brushed with distilled water and observed by SEM. One milliliter dentifrice solution (80 nm HA dentifrice, 300 nm HA dentifrice, ordinary dentifrice) was added to 50 ml potassium dichromate solution for 1, 14, and 28 d. The residual Chromium (Cr6+) concentration in the supernatant was measured by the diphenylcarbon phthalocyanine hydrazine method. The elemental constitution in the precipitate was detected by EDS. The Kruskal-Wallis test was used to analyze surface mineralization and different plugging rates of dentinal tubules. The absorption capacity of dentifrices were also evaluated by the Kruskal-Wallis test.
Results
The plugging rate in the HA dentifrice group was higher than that in the ordinary dentifrice group, and the 80 nm HA dentifrice group showed the best result. The atomic percentages of Ca and P of 80 nm dentifrice group on the surface of dentinal tubules were the highest. The 80 nm HA dentifrice group showed the best adsorption and stable effect of Cr6+, followed by the 300 nm HA dentifrice group. The 300 nm HA dentifrice and the ordinary dentifrice showed desorption phenomenon.
Conclusions
The dentifrice containing HA, especially the 80 nm HA dentifrice, exerts good dentinal tubule occlusion and surface mineralization effect. This dentifrice was also a good adsorbent of Cr6+.
Keywords: dentin hypersensitivity, nano-hydroxyapatite, dentifrice, heavy metal ion, chromium ion
Introduction
Dentin hypersensitivity (DH) refers to transient and severe pain caused by external stimuli such as heat, tactile, osmotic, or chemical stimulation, which cannot be diagnosed as arising from other dental defects or diseases.1–3 In recent years, the prevalence of dentin hypersensitivity has increased significantly. In a large European study, 42% of the patients reported suffered from DH.4 Prevalence of DH in Italian adults is up to 45%.5 A study found that 33.9% of young adults aged 24.08 years were diagnosed as DH, which suggests that DH tends to be in rejuvenation and needs to attract attention.6
Currently, in terms of the research and development of oral care products, different active ingredients such as fluoride,7,8 calcium oxalate,9 calcium carbonate–phosphate,10 strontium acetate,11 arginine,12,13 and hydroxyapatite (HA) can be added to a dentifrice to make a relieving DH dentifrice to block the open dentinal tubules which can reduce or inhibit the flow of tubule fluid and avoid stimulation of the pulp nerve endings. Among these ingredients, HA is the main inorganic component of dental hard tissue, which has excellent characteristics such as good biocompatibility, non-toxicity, and harmlessness.14,15 It can play a role in natural repair of open dentinal tubules, and it has become a research hot-spot for active ingredients added in an anti-sensitivity dentifrice.13
Environmental pollution such as heavy metal pollution is a major problem faced by many countries and it needs to be solved urgently.16–19 Chromium (Cr) with strong irritation and corrosiveness also has carcinogenic, reproductive toxic and mutagenic effects.20,21 Chromium (VI) (Cr6+) has higher toxicity and membrane penetration ability, and is more harmful to the human body. Therefore, Cr6+ is an important index for water pollution control. HA is an excellent adsorbent that has strong adsorption capacity, and it has a good adsorption effect on removing heavy metal ions from contaminated water or soil.22–26 Previous studies have shown that the 600 nm HA dentifrice causes good occlusion of dentinal tubules and adsorption of Cr6+.27
With the advance of nanotechnology, nano-hydroxyapatite (nano-HA) has emerged as a promising material and its use as a dentifricea nano-HA dentifrice could be interesting. Nano-HA possesses many excellent properties and new functions, and it also exerts many unique effects; thus, opening the way for search and manufacture of new materials with special functions.
To date, the application of nano-HA in oral care products has been mainly reported for achieving results such as remineralization of early enamel caries,28,29 and whitening and antibacterial effect.30,31 However, there are few reports regarding the anti-sensitivity effect of nano-HA. Studies in which nano-HA is added to a dentifrice and used as a carrier to treat dentin hypersensitivity are mostly clinical research.32,33 In addition, previous studies mainly focused on whether a single-particle size HA dentifrice could block the dentinal tubules, and there is a lack of a comparative study for assessing the anti-sensitivity effect of a dentifrice containing different particle sizes of HA. Nano-HA are widely used in the area of heavy metal ion adsorbent.25 However, studies assessing the adsorption effect of a nano-HA dentifrice on heavy metal ions have been rarely reported.
Therefore, based on the excellent properties of nano-HA, we hypothesized that nano-HA dentifrice has better ability on plugging dentinal tubules and adsorption effect of Cr6+. The stability level and the relationship between adsorption and HA particle size was also assessed.
Materials and methods
Ethics statement
The protocol was approved by the Academic and Medical Ethics Committee of Stomatological Hospital of Southern Medical University (approval number: 2011014).
Preparation of dentin discs
Fifty premolars and fifty molars extracted due to orthodontic treatment or periodontal disease were collected after obtaining written informed consent in the Department of Oral and Maxillofacial Surgery, Stomatological Hospital of Southern Medical University. The sample collection criteria were presence of a complete crown, absence of caries, absence of a crack, and no repair therapy. The soft tissue around the tooth was removed and scrubbed clean, and then it was stored in 10% neutral formaldehyde solution.
The preparation of dentin discs was performed according to a previous paper.27 Occlusal enamel layer was removed by turbine drill cooled with cold water. Dentine discs were obtained by cutting parallel to the occlusal surface at about 1.6 mm below the occlusal dentino-enamel junction. The occlusal side of samples was stepwisely polished with silicon carbide abrasive papers to form about 1.5 mm thickness of homogeneous, flat, and smooth surface. Dentine discs were etched with 10% citric acid for 2 mins to clear the smear layer for exposing the dentinal tubules, thus preparing dentine discs suitable for studying DH.
Preparation of artificial saliva
Artificial saliva was prepared as follows:34 NaCl (0.4 g), KCl (0.4 g), NaH2PO4 (0.1 g), Na2S·2H2O (0.005 g), Urea (1 g), CaCl2·2H2O (0.795 g), deionized water (1000 ml). The pH value of the solution was adjusted to 6.8 (37°C) using 0.01% HCl and 0.05% NaOH.
Preparation of a dentifrice
The calcium carbonate dentifrice and the HA/calcium carbonate dentifrice containing 3% (w/w) of HA with particle sizes of 300 and 80 nm were made by the Foshan Engineering Center for Oral Care Products (Guangdong, China) according to national standards (GB/T 8372–2017 Dentifrice).
Analysis of dentinal tubule occlusion
Ten dentin discs were randomly selected from 50 premolar dentin discs as untreated group for the energy dispersive spectrometer (EDS) test. The remaining 40 dentin discs were divided into the following 4 groups: the blank group treated with distilled water, the control group treated with a calcium carbonate dentifrice, and the experimental groups 1 and 2 treated with an 80 nm HA/calcium carbonate dentifrice and a 300 nm HA/calcium carbonate dentifrice, respectively.
50 molar dentine discs were divided into similar groups and parallel experiments were carried out.
The experimental method was based on a previous literature.27 The dentin discs were fixed and brushed with a 0.2 g dentifrice sample immersed in distilled water using an electric toothbrush (Oral B, USA) twice a day for 2 mins, for 7 days. The dentin slices were then divided into two parts; eight dentin discs were randomly selected from one half of the discs for air drying, glutaraldehyde fixation, gold spraying, and then analyzed by scanning electron microscope (SEM, S-3700N, Hitachi, Japan) under the condition of accelerating voltage of 10 kV and resolution of 3 nm. EDS was also used to detect and analyze the elements on the surface of dentin discs.
The other half of the dentin discs were subjected to mechanical brushing with distilled water twice a day for 2 mins, for 7 days. Eight dentin discs were then randomly selected for SEM detection.
All dentin discs were immersed in fresh artificial saliva and stored in a 37°C constant temperature box during the process. The entire experimental operation process was carried out by the same operator.
Cr6+ adsorption effect of the dentifrice
The samples in this study were divided into the following three groups: the blank group treated with a common calcium carbonate dentifrice, the experimental group 1 treated with an 80 nm HA/calcium carbonate dentifrice, and the experimental group 2 treated with a 300 nm HA/calcium carbonate dentifrice.
Potassium dichromate powder (K2Cr2O7) was prepared in a 100 μg/ml Cr6+ solution. The dentifrice was made into dentifrice suspensions with concentrations of 0.025, 0.050, 0.100, 0.200, 0.300, 0.400, 0.500, 0.600, 0.700, 0.800, 0.900, and 1.000 g/100 mL.
Twelve copies of 50 mL Cr6+ solution were considered as one group, and there were a total of three groups. One milliliter of dentifrice suspension of each concentration was added to the Cr6+ solution, stirred for 3 hrs, kept standing for 24 hrs, and centrifuged. The supernatant was absorbed, the absorbance value was measured by a 752N UV spectrophotometer, the concentration of residual heavy metal ions was determined, and the removal rate was calculated.
Stability analysis of Cr6+ adsorption effect of the HA dentifrice
The reaction system was configured according to the method described above, and the suspension was kept standing for 14 and 28 d. The residual heavy metal ion concentration was measured, and the stability of heavy metal ion adsorption capacity by 80 and 300 nm HA dentifrices was compared.
EDS analysis of Cr6+ solution precipitates
The Cr6+ solution sediment after centrifugation was dried to a powder form in a 120°C constant temperature drying oven, and EDS was used to test the powder elemental composition.
Result analysis
The dentinal tubule plugging rate (PR) calculated on the basis of the method used by Ahmed et al35,36 according to the following formula (1):
| (1) |
where N is the total number of dentinal tubules in the SEM image, S is the total area of the open area of dentinal tubules, and D is the mean diameter of dentinal tubules in the untreated group.
The mass concentration of Cr6+ in aqueous solution was calculated as follows:
| (2) |
where P is the concentration of Cr6+ in the reaction system, T is the absorbance of the sample, T0 is the absorbance of distilled water, B is the regression equation intercept, K is the slope of the regression equation, and V is the volume of the reaction system.
The Cr6+ removal rates by the dentifrice suspensions were calculated as follows:
| (3) |
where T is the removal rate, P is Cr6+ concentration in the water sample, V is the volume of the reaction system, V1 is the volume of the unreacted Cr6+ solution, and C is the initial concentration of Cr6+ solution.
Statistical analysis
The SPSS 23.0 (IBM, USA) package was used for statistical analyses. The Kruskal–Wallis test was used to analyze surface mineralization and different plugging rates of dentinal tubules treated with an ordinary dentifrice and a HA dentifrice. The absorption capacity of dentifrices was also evaluated by the Kruskal-Wallis test. The test level was set at P=0.05.
Results
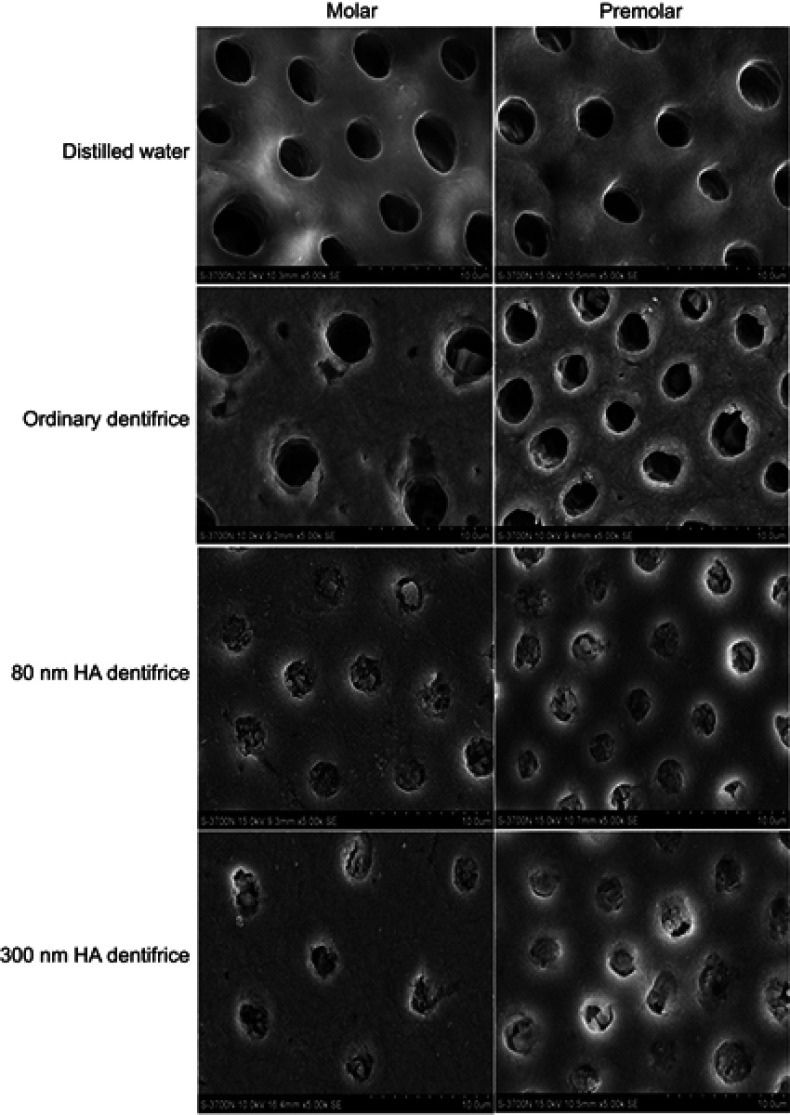
SEM observation of the occlusion effect of dentinal tubules treated with a dentifrice for 7 days (Figure 1)
Figure 1.
Dentinal tubule occlusion of dentine discs after treatment with a dentifrice for 7 days (SEM, 5000×).
The blank group: the dentinal tubules were empty, blockage was not identified, and the boundary between the sediment and intertubular dentin was clear. The control group: most of the dentinal tubules were empty, a few dentinal tubules were found to have a few plugs that were blocky and were across the dentinal tubules, and the boundary between the sediment and intertubular dentin was clear. The 80 nm HA dentifrice group: the diameter of dentin tubules was reduced. Almost all dentinal tubules were blocked by dentifrice materials, the dentifrice materials were subtle and close-grained, and the boundary between the sediment and intertubular dentin was blurred. The 300 nm HA dentifrice group: the dentinal tubules were blocked with a dentifrice to 86.72%~96.80%. Some dentinal tubules still had voids and the blockage was not tight.
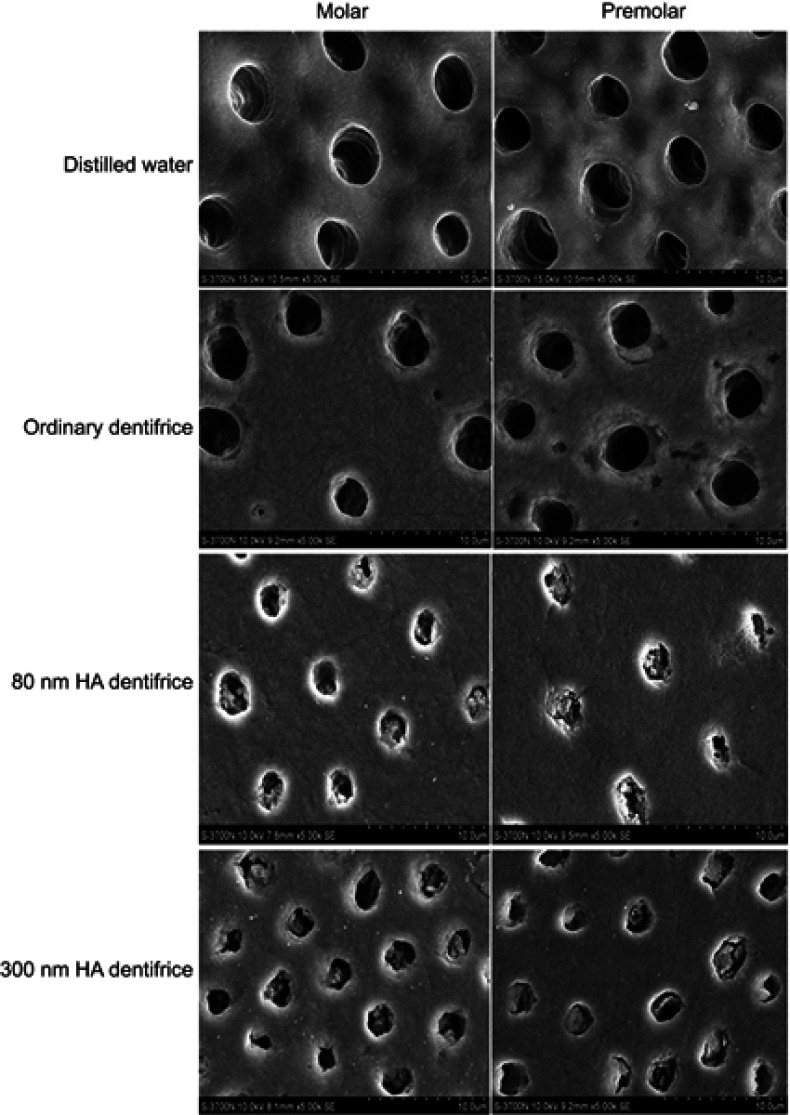
SEM observation of the occlusion effect of dentinal tubules after mechanical brushing with distilled water for 7 days (Figure 2)
Figure 2.
Dentinal tubule occlusion treated by mechanical brushing with distilled water for 7 days (SEM, 5000×).
The blank group: the dentinal tubules were still empty and blockage was not identified. The control group: almost all dentinal tubules were empty and a few plugs had adhered to the dentinal tubule wall. The plug was coarsely granular and the boundary between the sediment and intertubular dentin was clear. The 80 nm HA dentifrice group: the plug showed different degrees of loss, but it was still dense. The 300 nm HA dentifrice group: the plug showed different degrees of loss, some dentinal tubules were empty, and the sediment particles had adhered to the wall of dentin tubules or had blocked the dentinal tubules loosely.
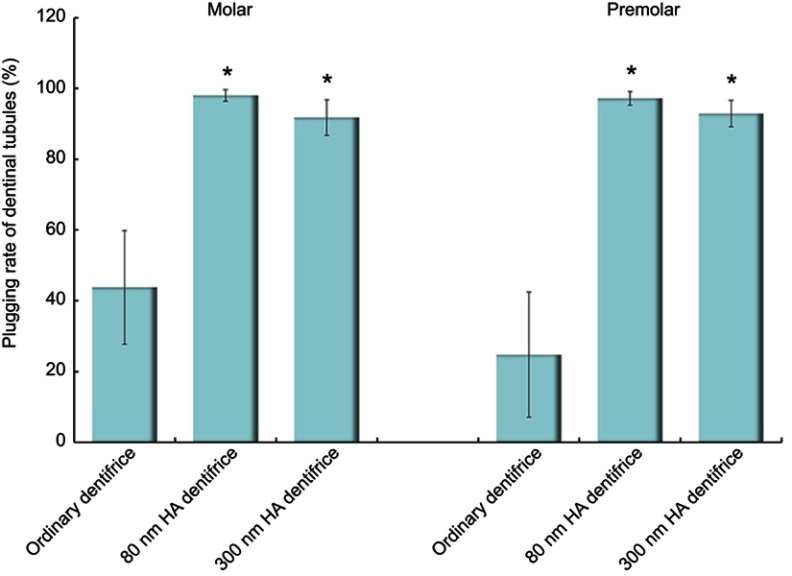
Comparison of the plugging rates of dentinal tubules treated with a dentifrice for 7 days
The results in the molar group are shown in Figure 3, and the differences among the three groups were statistically significant (χ2=59.430, P=0.000). It was considered that different kinds of dentifrices (the 80 nm HA dentifrice, the 300 nm HA dentifrice, and the ordinary dentifrice) exerted different blocking effects on dentinal tubules. According to the average rank, we concluded that the plugging rates in the HA dentifrice groups were significantly higher than those in the ordinary dentifrice group; in addition, we found that the 80 nm HA dentifrice group showed better results than the 300 nm HA dentifrice group. The premolar group showed the same results as the molar group (χ2=57.147, P=0.000).
Figure 3.
The plugging rates of dentinal tubules treated with a dentifrice for 7 days (Molar group: χ2=59.430, P=0.000; Premolar group: χ2=57.147, P=0.000; *P<0.05).
Comparison of the plugging rates of dentinal tubules after mechanical brushing with distilled water for 7 days
The results in the molar group are shown in Figure 4. After 7 days of washing with distilled water, the plugging rates of the 80 nm HA dentifrice, the 300 nm HA dentifrice, and the ordinary dentifrice were statistically significantly different (χ2=52.585, P=0.000). According to the average rank, the plugging rates in the 80 nm and 300 nm HA dentifrice groups were significantly higher than that in the ordinary dentifrice group. Although the plugging rate in the 80 nm HA dentifrice group was decreased to some extent, the plugging effect was still more than 90%, and this group showed a better continuous plugging effect than the 300 nm HA dentifrice group. The premolar group showed the same results as the molar group (χ2=56.314, P=0.000).
Figure 4.
Dentinal tubules after mechanical brushing with distilled water for 7 days (Molar group: χ2=52.585, P=0.000; Premolar group: χ2=56.314, P=0.000; *P<0.05).
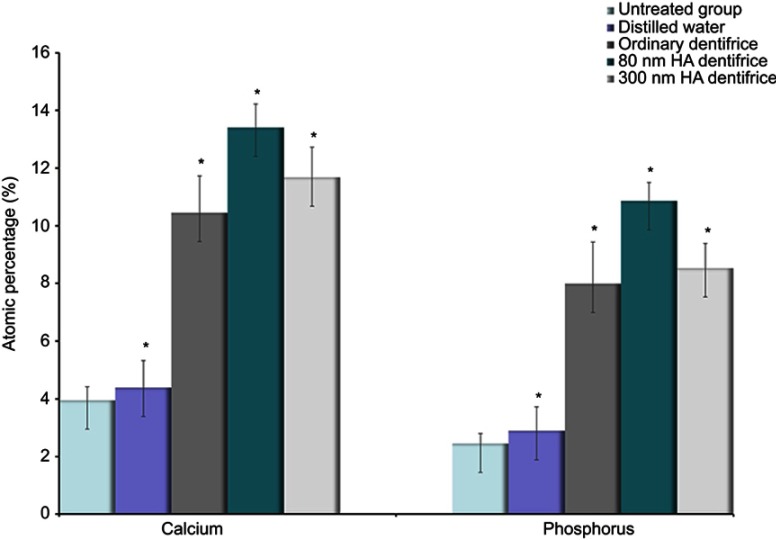
Comparison of surface mineralization of dentinal tubules treated with a dentifrice
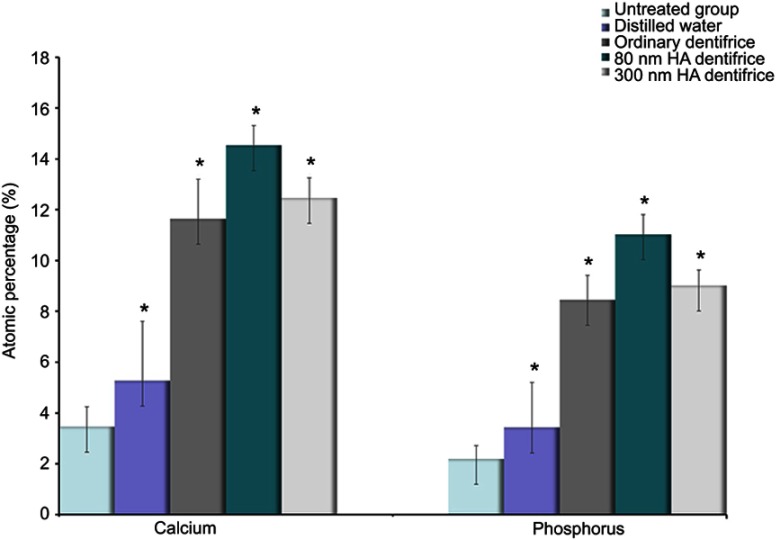
Ca and P elements, which are the main components that reflect the degree of mineralization, were statistically analyzed. The test results for the atomic percentage of Ca and P in molar group are shown in Figure 5. It suggests that there were statistically significant differences in remineralization among groups (Ca: χ2=33.244, P=0.000; P: χ2=33.518, P=0.000). As observed in Figure 6, similar results were obtained in the premolar group (Ca: χ2=33.637, P=0.000; P: χ2=33.855, P=0.000). On the basis of the mean rank, it was further inferred that the order of dentine mineralization degree was as follows: the 80 HA dentifrice group > the 300 HA dentifrice group > the ordinary dentifrice group > the distilled water group.
Figure 5.
Atomic percentages of calcium and phosphorus in dentin in the molar group (Ca: χ2=33.244, P=0.000; P: χ2=33.518, P=0.000; *P<0.05).
Figure 6.
Atomic percentages of calcium and phosphorus in dentin in the premolar group (Ca: χ2=33.637, P=0.000; P: χ2=33.855, P=0.000; *P<0.05).
Comparison of Cr6+ removal from water
Under the condition of constant concentration of Cr6+ solution, the concentration of residual Cr6+ in the solution decreased gradually with an increase in the concentration of dentifrice suspension added. The removal rate of Cr6+ was highest in the 80 nm HA dentifrice group, and it was up to 49.29%, which was followed by 44.72% in the 300 nm HA dentifrice group and 34.36% in the ordinary dentifrice group (Figure 7A).
Figure 7.
Adsorption capacity of a dentifrice. (A) Adsorption capacity of a dentifrice for Cr6+ at 1 day. (B) Adsorption of Cr6+ by the 80 nm HA dentifrice at 1, 14, and 28 days. (C) Adsorption of Cr6+ by the 300 nm HA dentifrice at 1, 14, and 28 days. (D) Adsorption of Cr6+ by an ordinary dentifrice at 1, 14, and 28 days.
Figure 7B shows that the adsorption of Cr6+ in the 80 nm HA dentifrice group did not change significantly over time. The adsorption curves at 14 and 28 days fluctuated around the adsorption curve at 1 day, with a small range.
Whether 14 or 28 days, in the concentration area of 0.025–0.7 g/100 mL, desorption phenomenon was noted; and Cr6+ removal by the 300 nm HA dentifrice decreased gradually over time. However, the adsorption capacity in the concentration area of 0.8–1 g/100 mL did not change significantly compared with that in the standing 1 day group and the removal rate fluctuated around 44.72% (Figure 7C).
Cr6+ removal by the ordinary dentifrice was unstable, and it increased over time at a low concentration (0.025–0.5 g/100 mL). However, at a high concentration (0.7–1 g/100 mL), there was no obvious change (Figure 7D).
EDS analysis of a dentifrice before and after sorption of Cr6+
The existence of chromium in the precipitates from dentifrice sorption was detected by EDS (Figure 8), which further confirmed that Cr6+ in water was adsorbed and precipitated by a dentifrice.
Figure 8.
EDS spectra before and after dentifrice adsorbed Cr6+. The horizontal axis represents the value of the voltage (keV) at which different elements can be excited. The findings comparing between before/after diagrams for each group showed that there was chromium in the powder which was further confirmed that the HA dentifrice could adsorb Cr6+.
Discussion
Characteristics of HA and principle of plugging dentinal tubules
HA is a major component of human bones and teeth.37,38 Artificial HA is widely recognized as a non-toxic and safe biological material that has a crystal structure similar to natural bone minerals and human teeth, and it shows good potential for natural sealing of dentinal tubules and anti-dentin sensitivity. On the other hand, natural HA in living organisms mainly exists in the form of nanocrystals. Compared with micrometer grade HA, nano-HA has better biological properties and physicochemical properties. It is more similar to natural HA in human body in terms of morphology, crystal structure synthesis and crystal degree, and better biocompatibility and bioactivity.39,40 Because of the quantum size effect, surface effect, and macroscopic quantum tunneling effect, nano-HA has many unique properties, such as a large surface area, many active sites, high surface reaction activity, and strong adsorption ability.41,42 This opens up a new way for the study of anti-sensitivity.
A perfect anti-sensitivity dentifrice should not only reduce the fluid flow in dentinal tubules, but it should also meet the challenge of saliva flushing and various complex conditions in the oral environment, and it should play the role of a persistent plug of dentinal tubules.43 Therefore, it is necessary to evaluate whether the dentifrice plays the function of plugging dentinal tubules in a simulated oral environment. During the process of the whole experiment, after brushing, dentin discs were stored in artificial saliva, which simulated the main minerals of saliva. It also simulated the temperature of the oral environment (37°C), pH value (6.8), and replaced fresh artificial saliva every day to maintain all concentrations of mineral ions in the solution to achieve a similar effect as the oral environment to the greatest possible extent. In this study, it was found that a dentifrice containing different particle sizes of HA exerted a good plugging effect in both the premolar group and the molar group. The plugging rate was significantly higher than that in the ordinary dentifrice group, with a plugging rate as high as 90.31–98.81%. This could be due to the fact that HA is the main inorganic component of dental hard tissue and about 70% of the minerals in dentin are HA crystals. Also, the crystal structure of HA particles is the same as that of HA in dentin, which has good biocompatibility and bioactivity.44,45 Homogeneous attraction characteristics and binding force with some kind of crystalline form or substance may play the biological role of natural restoration of dentin while blocking the dentinal tubules.
The blocking effect of the 80 nm HA dentifrice on dentinal tubules was better than that of the 300 nm HA dentifrice, which was in line with our hypothesis. The good plugging effect of the nanoscale HA dentifrice may have resulted from the fact that the nanometer size of nano-HA relative to the dentinal tubule diameter (about 2.99–3.50 μm) is very small and it can easily enter the dentinal tubules, while the micron grade HA particle size is bigger and entry into the dentinal tubules is difficult. Further, 25% of the volume of dentinal tubules is occupied by free liquids, whose components and properties are similar to those of body fluids. The micron HA crystal has a large size and a small total contact surface area with the solution; therefore, degradation is difficult and a relatively high concentration of calcium-rich and phosphate-rich environment is hard to produce. When it is precipitated out in the form of ions and partially deposited on the surface of dentin, the concentrations of calcium and phosphorus in saliva drop rapidly and revert to the unsaturated state; thus delaying the continuous entry of calcium and phosphorus into the surface of dentin and making it difficult to carry out chemical deposition in tubules. Secondly, nano-HA has a good adhesion force on the dentine surface. Due to the “nano effect”, the number of nanoparticles per unit mass surface area is significantly greater than the number of micron particles. The number of atomics on the surface and the ratio of the total number of atoms increases sharply with a decrease in the particle size, which makes the atoms more focused on the surface of nanoparticles. Thus, the number of atoms increases on the surface of nano-HA, which makes the coordination number insufficient, and the surface of atoms has high surface chemical activity and easily binds with protein atoms in dentin tubules.46 Nano-HA can form a stable suspension in the liquid-phase dispersion system; therefore, it can contact the surface of dentin in large quantities and play a deposition role. At the same time, nano-HA has strong remineralization ability due to its nanomaterial properties.47,48
Continuous plugging effect of the HA dentifrice
Studies have proved that mechanical brushing by a toothbrush has a strong abrasion effect on the teeth.49,50 HA is self soluble in an aqueous solution,51 and the salivary components can wash and dissolve the precipitates or mineralized products on the surface of dentin.52,53 A highly efficacious anti-sensitivity dentifrice should be able to resist all kinds of adverse external environments, should have a good continuous closure effect on the dentinal tubules, and should exert a long-lasting anti-sensitivity effect.
After 7 days of mechanical scrubbing with distilled water, we found that the surface sediments were lost to varying degrees in the HA dentifrice group, and the loss rate in the 300 nm HA dentifrice group was higher than that in the 80 nm HA dentifrice group. In the 80 nm HA dentifrice group, the plugging was tight and it caused continuous relief and anti-sensitivity effect. Individual dentinal tubules in the 300 nm HA dentifrice group were empty and the plugs were partially loose. In the ordinary dentifrice group, the dentinal tubules were almost empty. This may be due to the fact that the plugging effect of the HA dentifrice on dentin tubules was not only manifested as surface sealing, but also because of the fact that emboli in the dentin tubules were deep enough to resist the daily brushing behavior. The solubility of 300 nm HA in a solution is low51 and calcium and phosphorus ions can be released at the same time of dissolution, which changes the unsaturated state of calcium and phosphorus ions in the solution into a saturated state. Subsequently, calcium ions combine with the free radicals of proteins in the dentin to promote the remineralization of the exposed dentin. Then, calcium ions combine with free radicals of proteins in the dentin to facilitate the remineralization of the exposed dentin.
Previous studies have shown that the Gibbs free energy of nano-HA has the largest negative value (−5.17 kJ/mol) compared with a variety of calcium phosphate salts, which ensures its stronger ability to maintain the supersaturated essential state in artificial saliva and increase the migration ability of Ca2+.43 Mechanical brushing for 7 days did not form the boundary between the precipitates and intertubular dentin in the HA dentifrice group became clear, which also showed that the HA dentifrice group showed further mineralization along with blocking of the dentinal tubules.43
The HA dentifrice promotes dentin mineralization
The results of this study showed that all the experimental groups showed different degrees of mineralization than those before the experiment. EDS showed that after brushing with a dentifrice for 7 days, calcium and phosphorus elements increased to different degrees. The HA dentifrice groups showed better mineralization effect than the ordinary dentifrice group and the distilled water group, and the 80 nm HA dentifrice group showed the highest degree of mineralization.
The results of the present study showed that the percentages of calcium and phosphorus on the dentin surface in the distilled water group were higher than those in the untreated group. The mineral in salivary deposit on dentinal tubules is one of the most important reasons.54
Adsorption ability and principle of Cr (VI) with the HA dentifrice
According to the Ministry of Environmental Protection, fourteen key provinces of China make up the biggest share of the country’s heavy metal discharges, including Cr (74%).55 Its more common oxidation states are tri valent and hexa valent.56 Cr6+ with a high degree of toxicity, which have toxicity for living organism and harmful for human health, can damage multiple organs with small exposures.57 Various physical and chemical treatment methods containing adsorption, ion exchange, chemical precipitation, and biological method have been used worldwide for removing heavy metals from polluted solution.58–60 Compared with the above methods, adsorption has been widely used in the removal of heavy metals in polluted water because of its high efficiency, high selectivity, high cation adsorption capacity, and low cost.61 HA is good at ion exchange. HA has been proven to be efficient for heavy metal cations removal from aqueous solution on account of its high adsorption capacity, low solubility and high stability under oxidizing and reducing conditions, and less residue production.62 Nano-HA belongs to hexagonal crystal system. Nano-HA is significantly different in performance from the traditional micrometer grade HA of the same composition due to its excellent micro-structure, and special physical and chemical properties. Recent years, nano materials have become an adsorbent research hotspot in the field of wastewater treatment, owing to their high specific surface area, slow bio-degradation, excellent bio-compatibility, and good mechanical stability.63
Dentifrice suspensions had different adsorption abilities for chromium ion solution, and among them, the 80 nm HA dentifrice group showed the best adsorption effect, followed by the 300 nm HA dentifrice group. The absorptivity in the ordinary dentifrice group for chromium ion solution was 34.36% (171.8 mg/g), which may be the basic component of an ordinary dentifrice that has particular adsorption ability for chromium ions.
Adsorption capacity of the 80 nm dentifrice for heavy metal ions was better than that of the 300 nm HA dentifrice, which may be due to nano-HA with the surface effect of nanoparticles and then the ratio of surface atomic number and total number of atoms increased sharply. As the number of surface atoms increased, the insufficient coordination number of surface atoms led to a large number of suspended bonds, which increased the surface area and caused extremely high surface chemical activity.64 Therefore, the contact area between nano-HA and heavy metal ions increased and serious unsaturation of surface atomic coordination number also increased the binding ability of heavy metal ions.
On the basis of the results, we further calculated that the adsorption capacity of the 80 nm HA dentifrice was about 22.85 mg/g, which was not prominently outstanding compared with the 300 nm HA dentifrice. It is worth considering that since the chromium ion adsorption capacity of the micron grade HA dentifrice was not different from that of the nano-HA dentifrice and current studies have reported that nanoparticles have potential toxic effects on cells,65,66 therefore, in practical application, we should not blindly tout the nanomaterials. This also reduces the technical requirements and decreases the cost of the production process; thus, ensuring promotion of products.
Interestingly, previous studies have suggested that the removal effect of HA particles on Cr6+ in wastewater was 23.54 mg/g. However, the results of the present study indicated that the adsorption capacity of the 80 and 300 nm HA dentifrices for Cr6+ was as high as 223.6–246.45 mg/g. Even after ignoring the adsorption rate of the dentifrice, the adsorption efficiency of the HA dentifrice was still as high as 51.8–74.65 mg/g, which was far higher than that of HA particles. This suggests that HA may have a synergistic effect with the original dentifrice ingredients in the adsorption of Cr6+.
By comparing the adsorption capacity of dentifrice solutions on Cr6+ at 1, 14, and 28 days, the results indicated that 80 nm HA dentifrice is a good Cr6+ adsorption material and a green adsorbent while 300 nm HA dentifrice and the blank dentifrice was not stable on Cr6+ adsorption.
Limitations of the study
In this study, the plugging stability was measured for only one week, but its further persistence without additional toothpaste use is uncertain. Also, some factors that may interfere with the plugging ability such as salivary flow, mastication force, acid challenge, etc are not discussed in this study. In the future, we will focus on these aspects on further study.
Although the present study found that the HA dentifrice had a strong adsorption effect on Cr6+, there are many kinds of wastewater in the environment and most of them exist in mixed form. Therefore, it remains to be studied whether the HA dentifrice can adsorb and remove other heavy metal ions such as Cu2+, Zn2+, Pb2+, Cd2+, Ni2+, Cr2+. Whether the adsorption capacity of heavy metal ions in mixed form is mutually affected still needs to be discussed.
Conclusion
The HA dentifrice had better plugging and mineralization effects on the dentinal tubules, especially the 80 nm HA dentifrice. The 80 and 300 nm HA dentifrices had strong adsorption ability for Cr6+ in simulated wastewater. The adsorption efficiency of the 80 nm HA dentifrice was the highest and it increased with an increase in the concentration of the dentifrice suspension. The adsorption effect of the 80 nm HA dentifrice on Cr6+ was stable. The adsorption effects of the 300 nm HA dentifrice and the ordinary dentifrice on Cr6+ were unstable, and they included desorption or increase in the adsorption rate over time.
Acknowledgments
The study was supported by the Guangzhou Municipal Science & Technology Projects (No. 201707010199) and Stomatological Hospital of Southern Medical University Research and Development Program (No. PY2018020).
Disclosure
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Addy M. Etiology and clinical implication of dentin hypersensitivity. Dent Clinic North Am. 1990;34:503–514. [PubMed] [Google Scholar]
- 2.Addy M. Dentine hypersensitivity: new perspectives on an old problem. Int Dent J. 2002;52:367–375. doi: 10.1002/j.1875-595X.2002.tb00936.x [DOI] [Google Scholar]
- 3.Canadian Advisory Board on Dentin Hypersensitivity. Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. J Can Dent Assoc. 2003;69:221–226. [PubMed] [Google Scholar]
- 4.West NX, Sanz M, Lussi A, Bartlett D, Bouchard P, Bourgeois D. Prevalence of dentine hypersensitivity and the study of associated factors: a European population-based cross-sectional study. J Dent. 2013;41(10):841–851. doi: 10.1016/j.jdent.2013.07.017 [DOI] [PubMed] [Google Scholar]
- 5.Guerra F, Corridore D, Cocco F, et al. Oral health sentinel-based surveillance: a pilot study on dentinal hypersensitivity pain. ClinTer. 2017;168(5):e333–e337. [DOI] [PubMed] [Google Scholar]
- 6.Alcântara PM, Barroso NFF, Botelho AM, et al. Associated factors to cervical dentin hypersensitivity in adults: a transversal study. BMC Oral Health. 2018;18(1):155. doi: 10.1186/s12903-018-0507-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parkinson CR, Hughes N, Hall C, Whelton H, Gallob J, Mason S. Three randomized clinical trials to assess the short-term efficacy of anhydrous 0.454% w/w stannous fluoride dentifrices for the relief of dentin hypersensitivity. Am J Dent. 2016;29(1):25–32. [PubMed] [Google Scholar]
- 8.Parkinson C, Hughes N, Jeffery P, et al. The efficacy of an experimental dentifrice containing 0.454% w/w stannous fluoride in providing relief from the pain of dentin hypersensitivity: an 8-week clinical study. Am J Dent. 2013;26:25A–31A. [PubMed] [Google Scholar]
- 9.Lynch MC, Perfekt R, McGuire JA, et al. Potassium oxalate mouthrinse reduces dentinal hypersensitivity: a randomized controlled clinical study. J Am Dent Assoc. 2018;149(7):608–618. doi: 10.1016/j.adaj.2018.02.027 [DOI] [PubMed] [Google Scholar]
- 10.Medvecky L, Stulajterova R, Giretova M, et al. Effect of tetracalcium phosphate/monetite toothpaste on dentin remineralization and tubule occlusion in vitro. Dent Mater. 2018;34(3):442–451. doi: 10.1016/j.dental.2017.11.022 [DOI] [PubMed] [Google Scholar]
- 11.Arnold WH, Prange M, Naumova EA. Effectiveness of various toothpastes on dentine tubule occlusion. J Dent. 2015;43(4):440–449. doi: 10.1016/j.jdent.2015.01.014 [DOI] [PubMed] [Google Scholar]
- 12.Kumar S, Thomas BS, Gupta K, Guddattu V, Alexander M. Iontophoresis and topical application of 8% arginine-calcium carbonate to treat dentinal hypersensitivity. Niger J Clin Pract. 2018;21(8):1029–1033. doi: 10.4103/njcp.njcp_341_17 [DOI] [PubMed] [Google Scholar]
- 13.Anand S, Rejula F, Sam JV, Christaline R, Nair MG, Dinakaran S. Comparative evaluation of effect of nano-hydroxyapatite and 8% arginine containing toothpastes in managing dentin hypersensitivity: double blind randomized clinical trial. Acta Medica (Hradec Kralove). 2017;60(3):114–119. doi: 10.14712/18059694.2018.3 [DOI] [PubMed] [Google Scholar]
- 14.Driessens F. Mineral aspect of dentistry. Monogr Oral Sci. 1982;10:1–215. [PubMed] [Google Scholar]
- 15.Leegeros R. Calcium phosphates in oral biology and medicine. Monogr Oral Sci. 1991;15(15):1–201. [PubMed] [Google Scholar]
- 16.Fayiga AO, Ipinmoroti MO, Chirenje T. Environmental pollution in Africa. Environ Dev Sustain. 2018;20(1):41–73. doi: 10.1007/s10668-016-9894-4 [DOI] [Google Scholar]
- 17.Sodango TH, Li X, Sha J, Bao Z. Review of the spatial distribution, source and extent of heavy metal pollution of soil in China: impacts and mitigation approaches. J Health Pollut. 2018;8(17):53–70. doi: 10.5696/2156-9614-8.17.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shifaw E. Review of heavy metals pollution in China in agricultural and urban soils. J Health Pollut. 2018;8(18):180607. doi: 10.5696/2156-9614-8.18.180607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emmanuel AY, Jerry CS, Dzigbodi DA. Review of environmental and health impacts of mining in Ghana. J Health Pollut. 2018;8(17):43–52. doi: 10.5696/2156-9614-8.17.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linos A, Petralias A, Christophi CA, et al. Oral ingestion of hexavalent chromium through drinking water and cancer mortality in an industrial area of Greece – an ecological study. Environ Health. 2011;10:50. doi: 10.1186/1476-069X-10-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeidler-Erdely PC, Battelli LA, Salmen-Muniz R, et al. Lung tumor production and tissue metal distribution after exposure to manual metal ARC-stainless steel welding fume in A/J and C57BL/6J mice. J Toxicol Environ Health A. 2011;74:728–736. doi: 10.1080/15287394.2011.556063 [DOI] [PubMed] [Google Scholar]
- 22.Xing J, Hu T, Cang L, Zhou D. Remediation of copper contaminated soil by using different particle sizes of apatite: a field experiment. SpringerPlus. 2016;5(1):1182. doi: 10.1186/s40064-016-2492-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Y, Qiu W, Yu Z, Song Z. Toxic effect of cadmium adsorbed by different sizes of nano-hydroxyapatite on the growth of rice seedlings. Environ Toxicol Pharmacol. 2017;52:1–7. doi: 10.1016/j.etap.2017.03.005 [DOI] [PubMed] [Google Scholar]
- 24.Li Q, Chen X, Chen X, Jin Y, Zhuang J. Cadmium removal from soil by fulvic acid-aided hydroxyapatite nanofluid. Chemosphere. 2019;215:227–233. doi: 10.1016/j.chemosphere.2018.10.031 [DOI] [PubMed] [Google Scholar]
- 25.Jing N, Zhou AN, Xu QH. The synthesis of super-small nano hydroxyapatite and its high adsorptions to mixed heavy metallic ions. J Hazard Mater. 2018;353:89–98. doi: 10.1016/j.jhazmat.2018.02.049 [DOI] [PubMed] [Google Scholar]
- 26.Iconaru S, Motelica-Heino M, Guegan R, Beuran M, Costescu A, Predoi D. Adsorption of Pb (II) ions onto hydroxyapatite nanopowders in aqueous solutions. Materials (Basel). 2018;11(11):2204. doi: 10.3390/ma11081451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan P, Shen X, Liu J, et al. Effects of dentifrice containing hydroxyapatite on dentinal tubule occlusion and aqueous hexavalent chromium cations sorption: a preliminary study. PLoS One. 2012;7(12):e45283. doi: 10.1371/journal.pone.0045283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grewal N, Sharma N, Kaur N. Surface remineralization potential of nano-hydroxyapatite, sodium monofluorophosphate, and amine fluoride containing dentifrices on primary and permanent enamel surfaces: an in vitro study. J Indian Soc Pedod Prev Dent. 2018;36(2):158–166. doi: 10.4103/JISPPD.JISPPD_142_17 [DOI] [PubMed] [Google Scholar]
- 29.Souza BM, Comar LP, Vertuan M, Fernandes Neto C, Buzalaf MA, Magalhães AC. Effect of an experimental paste with hydroxyapatite nanoparticles and fluoride on dental demineralisation and remineralisation in situ. Caries Res. 2015;49(5):499–507. doi: 10.1159/000438466 [DOI] [PubMed] [Google Scholar]
- 30.Park YD, Kim JH, Hwang KS. Research about tooth whitening and bacteria sticking capability with using dentifrice including nano-hydroxyapatite, sodium metaphosphate. Key Eng Mater. 2007;330–332:283–286. doi: 10.4028/www.scientific.net/KEM.330-332.283 [DOI] [Google Scholar]
- 31.Kim BI, Jeong SH, Jang SO, Kim KN. Tooth whitening effect of toothpastes conntaining nano-hydroxyapatite. Key Eng Mater. 2006;309–311:541–544. doi: 10.4028/www.scientific.net/KEM.309-311.541 [DOI] [Google Scholar]
- 32.Vano M, Derchi G, Barone A, Pinna R, Usai P, Covani U. Reducing dentine hypersensitivity with nano-hydroxyapatite toothpaste: a double-blind randomized controlled trial. Clin Oral Investig. 2018;22(1):313–320. doi: 10.1007/s00784-017-2113-3 [DOI] [PubMed] [Google Scholar]
- 33.Vano M, Derchi G, Barone A, Covani U. Effectiveness of nano-hydroxyapatite toothpaste in reducing dentin hypersensitivity: a double-blind randomized controlled trial. Quintessence Int. 2014;45(8):703–711. doi: 10.3290/j.qi.a32240 [DOI] [PubMed] [Google Scholar]
- 34.ISO TR. 10271 Dentistry-Determination of Tarnish and Corrosion of Metals and Alloys.Bern:International Standardization Organization in Switzerland, 1993;5:13P. [Google Scholar]
- 35.Ahmed TR, Mordan NJ, Gilthorpe MS, Gillam DG. In vitro quantification of changes in human dentine tubule parameters using SEM and digital analysis. J Oral Rehabil. 2005;32(8):589–597. doi: 10.1111/j.1365-2842.2005.01473.x [DOI] [PubMed] [Google Scholar]
- 36.Lee SY, Kwon HK, Kim BI. Effect of dentinal tubule occlusion by dentifrice containing nano-carbonate apatite. J Oral Rehabil. 2008;35(11):847–853. doi: 10.1111/j.1365-2842.2008.01876.x [DOI] [PubMed] [Google Scholar]
- 37.Driessens FC. Mineral aspect of dentistry. Monogr Oral Sci. 1982;10:1–215. [PubMed] [Google Scholar]
- 38.LeGeros RZ. Calcium phosphates in oral biology and medicine. Monogr Oral Sci. 1991;15:1–201. [PubMed] [Google Scholar]
- 39.Huang SB, Gao SS, Yu HY. Effect of nano-hydroxyapatite concentration on remineralization of initial enamel lesion in vitro. Biomed Mater. 2009;4(3):034104. doi: 10.1088/1748-6041/4/3/034104 [DOI] [PubMed] [Google Scholar]
- 40.Li Y, de Groot K, de Wijin J, Klein CPAT, Meer SVD. Morphology and composition of nanograd grade calcium phosphate needle-like crystals formed by simple hydrothermal treatment. Mater Sci Mater Med. 1994;5(6–7):326–331. doi: 10.1007/BF00058956 [DOI] [Google Scholar]
- 41.Nguyen NH, Bai H. Photocatalytic removal of NO and NO2 using titania nanotubes synthesized by hydrothermal method. J Environ Sci. 2014;26:1180–1187. doi: 10.1016/S1001-0742(13)60544-6 [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, Jiang Z, Huang J, et al. Titanate and titania nanostructured materials for environmental and energy applications: a review. RSC Adv. 2015;5:79479–79510. doi: 10.1039/C5RA11298B [DOI] [Google Scholar]
- 43.Suge T, Ishikawa K, Kawasaki A, Yoshiyama M, Asaoka K, Ebisu S. Duration of dentinal tubule occlusion formed by calcium phosphate precipitation method: in vitro evaluation using synthetic saliva. J Dent Res. 1995;74(10):1709–1714. doi: 10.1177/00220345950740101301 [DOI] [PubMed] [Google Scholar]
- 44.Li SP. An Introduction to Biomedical Materials. Wuhan: Wuhan University of Technology Press; 2000. [Google Scholar]
- 45.Aoki H. Hydroxyapatite. Tokyo: Ishiyako Publishers, Inc.; 1999. [Google Scholar]
- 46.Geiger S, Matalon S, Blasbalg J, Tung M, Eichmiller FC. The clinical effect of amorphous calcium phosphate (ACP) on root surface hypersensitivity. Oper Dent. 2003;28(5):496–500. [PubMed] [Google Scholar]
- 47.Daas I, Badr S, Osman E. Comparison between fluoride and nano-hydroxyapatite in remineralizing initial enamel lesion: an in vitro study.J. Contemp Dent Pract. 2018;19(3):306–312. doi: 10.5005/jp-journals-10024-2258 [DOI] [PubMed] [Google Scholar]
- 48.Roza H, Reza HH, Farid A, Mohammad T. The effect of nano-hydroxyappatite solution on the permanent tooth remineralization following exposure to soft beer (in situ). J Dent Med. 2015;27(4):171–178. [Google Scholar]
- 49.Kodaka T, Kobori M, Hirayama A, Abe M. Abrasion of human enamel by brushing with a commercial dentifrice containing hydroxyapatite crystals in vitro. J Electron Microsc (Tokyo). 1999;48(2):167–172. doi: 10.1093/oxfordjournals.jmicro.a023663 [DOI] [PubMed] [Google Scholar]
- 50.Wiegand A, Wegehaupt F, Werner C, Attin T. Susceptibility of acid-softened enamel to mechanical wear-ultrasonication versus toothbrushing abrasion. Caries Res. 2007;41(1):56–60. doi: 10.1159/000096106 [DOI] [PubMed] [Google Scholar]
- 51.Pan HB, Darvell BW. Solubility of hydroxyapatite by solid titration at pH 3–4. Arch Oral Boil. 2007;52(7):618–624. doi: 10.1016/j.archoralbio.2006.12.007 [DOI] [PubMed] [Google Scholar]
- 52.Gandolfi MG, Silvia F, Pashley DH, Gasparotto G, Carlo P. Calcium silicate coating derived from Portland cement as treatment for hypersensitive dentine. J Dent. 2008;36(8):565–578. doi: 10.1016/j.jdent.2008.03.012 [DOI] [PubMed] [Google Scholar]
- 53.Arrais CA, Micheloni CD, Giannini M, Chan DC. Occluding effect of dentifrices on dentinal tubules. J Dent. 2003;31(8):577–584. [DOI] [PubMed] [Google Scholar]
- 54.Yoshiyama M, Noiri Y, Ozaki K, Uchida A, Ishikawa Y, Ishida H. Transmission electron microscopic characterization of hypersensitive human radicular dentin. J Dent Res. 1990;69(6):1293–1297. doi: 10.1177/00220345900690061401 [DOI] [PubMed] [Google Scholar]
- 55.Tan D, McGregor D. Analysis and reviews. China’s Soil Ten. 2016. [Google Scholar]
- 56.Rodríguez JP, Taber AB, Daszak P, et al. Globalization of conservation: a view from the south. Science. 2007;317(5839):755–756. doi: 10.1126/science.1145560 [DOI] [PubMed] [Google Scholar]
- 57.Mohanty M, Kumar Patra H. Effect of ionic and chelate assisted hexavalent chromium on mung bean seedlings (VignaRadiata l. Wilczek. Var k-851) during seedling growth. J Str Physiol Biochem. 2013;9(2):232–241. [Google Scholar]
- 58.Ni B, Huang Q, Wang C, Ni T, Sun J, Wei W. Competitive adsorption of heavy metals in aqueous solution onto biochar derived from anaerobically digested sludge. Chemosphere. 2019;219:351–357. doi: 10.1016/j.chemosphere.2018.12.053 [DOI] [PubMed] [Google Scholar]
- 59.Hong M, Yu L, Wang Y, et al. Heavy metal adsorption with zeolites: therole of hierarchical pore architecture. Chem Eng J. 2019;359:363–372. doi: 10.1016/j.cej.2018.11.087 [DOI] [Google Scholar]
- 60.Abdi G, Alizadeh A, Zinadini S, Moradi G. Removal of dye and heavy metal ion using a novel synthetic polyethersulfone nanofiltration membrane modified by magnetic graphene oxide/metformin hybrid. J Memb Sci. 2018;552:326–335. doi: 10.1016/j.memsci.2018.02.018 [DOI] [Google Scholar]
- 61.Luo X, Zeng J, Liu S, Zhang L. An effective and recyclable adsorbent for the removal of heavy metal ions from aqueous system: magnetic chitosan/cellulose microspheres. Bioresour Technol. 2015;194:403–406. doi: 10.1016/j.biortech.2015.07.044 [DOI] [PubMed] [Google Scholar]
- 62.Ali H, Khan E, Sajad MA. Phytoremediation of heavy metals-concepts and applications. Chemosphere. 2013;91(7):869–881. doi: 10.1016/j.chemosphere.2013.01.075 [DOI] [PubMed] [Google Scholar]
- 63.Tang WW, Zeng GM, Gong JL, et al. Impact of humic/fulvic acid on the removal of heavy metals from aqueous solutions using nanomaterials: a review. Sci Total Environ. 2014;15:468–469. [DOI] [PubMed] [Google Scholar]
- 64.Xu Y, Schwartz FW, Traina SJ. Sorption of Zn2+ and Cd2+ on hydroxyapatite surfaces. Environ Sci Technol. 1994;28(8):1472–1480. doi: 10.1021/es00057a015 [DOI] [PubMed] [Google Scholar]
- 65.Jovanović B, Whitley EM, Kimura K, Crumpton A, Palić D. Titanium dioxide nanoparticles enhance mortality of fish exposed to bacterial pathogens. Environ Pollut. 2015;203:153–164. doi: 10.1016/j.envpol.2015.04.003 [DOI] [PubMed] [Google Scholar]
- 66.Cao Y. The toxicity of nanoparticles to human endothelial cells. Adv Exp Med Biol. 2018;1048:59–69. doi: 10.1007/978-3-319-72041-8_4 [DOI] [PubMed] [Google Scholar]