Figure 3:
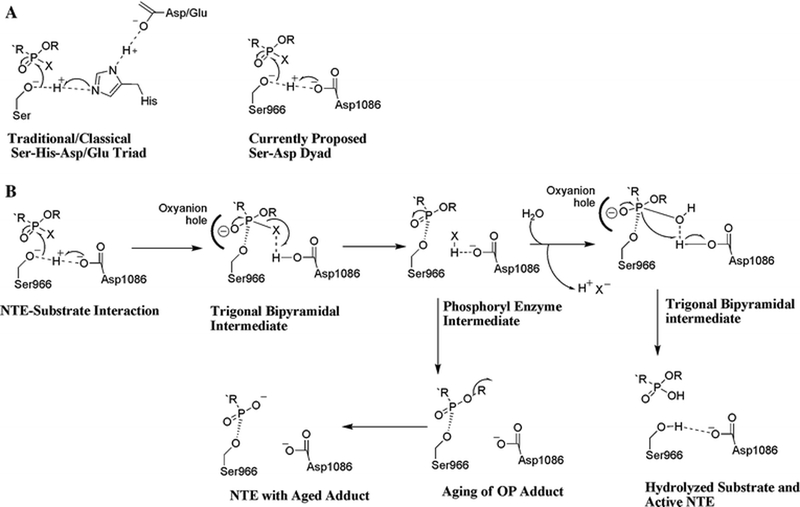
A.) Comparison of mechanisms of catalysis in a traditional Ser-His-Asp/Glu triad and our proposed Ser-Asp dyad for NTE. B.) Proposed mechanism for interactions of an OP ester with the NTE catalytic dyad. OP compounds are substrates that become mechanism-based inhibitors, because hydrolysis of the acyl-enzyme intermediate is usually slow. Moreover, this intermediate can undergo aging via net loss of an alkyl group to yield a negatively charged OP adduct on the active site Ser966 residue. It is currently postulated that aging of an OP adduct on NTE is the initiating step in the pathogenesis of OP compound-induced axonopathy.
