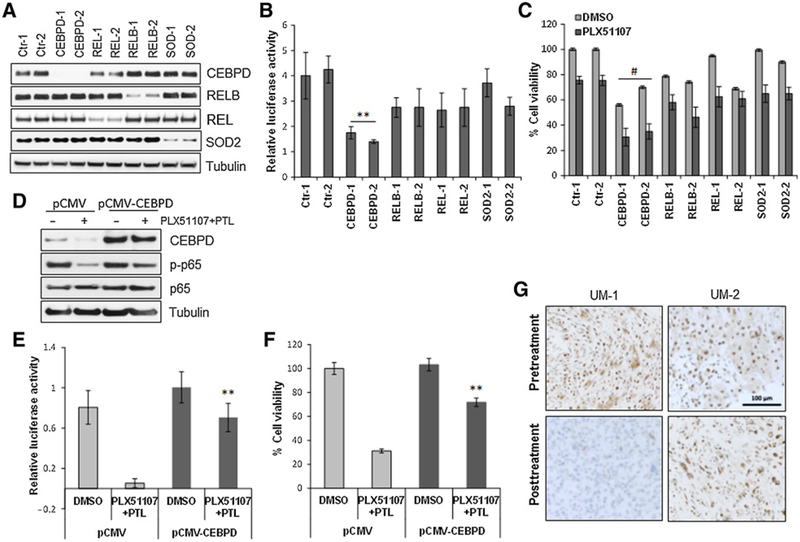
Figure 6.
Effects of gene silencing in the resistant phenotype. A, The indicated genes were silenced with two independent siRNA in the cell line R-92.1. Two nonspecific siRNAs were used as controls. B, siRNA-depleted cells were than transfected with an NF-κB-Luc vector and tested for NF-κB activity.**, P < 0.01. C, Cell viability assay of siRNA-transfected cells in the presence of 0.5 μmmol/L PLX51107 after 72 hours. Bars, mean ± SD. #, P < 0.05. D, R-92.1 cells were transfected with an empty vector (pCMV) or a CEBPD construct (pCMV-CEBPD). Cell lysates were subjected to immunoblotting using antibodies for CEBPD, p-p65, p65, and tubulin. E, NF-κB-Luc activity in vector and CEBPD-expressing cells with or without the combination treatment PLX51107 + PTL. **, P < 0.01. F, Viability assay of CEBPD-transfected cells after the combination treatment for 72 hours. Columns, mean ± SD of three independent experiments. **, P < 0.01. G, IHC analysis of CEBPD expression at baseline and after 2 weeks of treatment with PLX51107 in specimens from two representative patients with uveal melanoma. Suppression of CEBPD staining was observed after treatment in patient UM-1 who achieved prolonged stable disease lasting more than 1 year. Patient UM-2 had no significant changes in CEBPD expression and experienced disease progression.