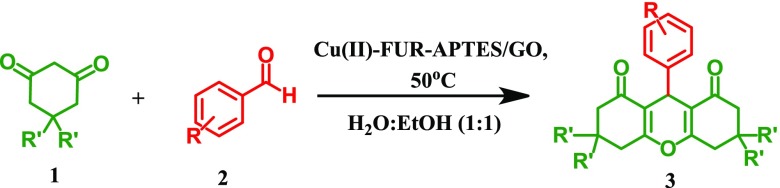
Table 2. Cu(II)-Fur-APTES/GO Catalyzed Synthesis of 1,8-Dioxo-octahydroxanthenesa.
| productc | R | R′ | time (min) | yieldb (%) |
|---|---|---|---|---|
| 3a | H | CH3 | 30 | 95 |
| 3b | 3-CH3 | CH3 | 35 | 93 |
| 3c | 3-Br | CH3 | 25 | 90 |
| 3d | 3-Cl | CH3 | 25 | 91 |
| 3e | 3-OCH3 | CH3 | 35 | 90 |
| 3f | 4-CH3 | CH3 | 40 | 89 |
| 3g | 4-OCH3 | CH3 | 50 | 89 |
| 3h | 4-NO2 | CH3 | 25 | 94 |
| 3i | 4-OH | CH3 | 50 | 86 |
| 3j | 4-Cl | CH3 | 30 | 92 |
| 3k | 4-CN | CH3 | 30 | 95 |
| 3l | 4-Br | CH3 | 25 | 92 |
| 3m | H | H | 30 | 94 |
| 3n | 3-CH3 | H | 35 | 92 |
| 3o | 3-Br | H | 25 | 94 |
| 3p | 3-Cl | H | 25 | 91 |
| 3q | 3-OCH3 | H | 35 | 89 |
| 3r | 2-CH3 | H | 50 | 85 |
| 3s | 4-CH3 | H | 40 | 90 |
| 3t | 4-NO2 | H | 30 | 95 |
Reaction conditions: aromatic aldehydes (1 mmol), dimedone or 1,3-cyclohexanedione (2 mmol), catalyst (20 mg), solvent (5 mL), temperature 50 °C.
Isolated yields.
Products were characterized by 1H and 13C NMR spectroscopy.