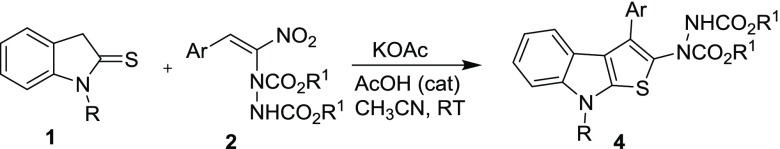
Table 2. Synthesis of Thienoindole 4 by Cascade Reaction of Indoline-2-thione 1 with Hydrazinonitroalkene 2a.
| entry | 1, R | 2, Ar | R1 | 3 or 4 | time | % yieldb |
|---|---|---|---|---|---|---|
| 1 | 1a, Me | 2a, 4-MeC6H4 | iPr | 4a | 4 d | 78 |
| 2 | 1a, Me | 2b, C6H5 | iPr | 4b | 4 d | 58 |
| 3 | 1a, Me | 2c, 4-OMeC6H4 | iPr | 4c | 2 d | 66 |
| 4 | 1a, Me | 2d, 4-OMeC6H4 | tBu | 4d | 3 d | 75 |
| 5 | 1a, Me | 2e, 4-ClC6H4 | iPr | 4e | 3 d | 66 |
| 6 | 1a, Me | 2f, 2-thienyl | iPr | 4f | 3 d | 40c |
| 7 | 1b, Et | 2a, 4-MeC6H4 | iPr | 4g | 8 d | 43c |
| 8 | 1c, nPr | 2a, 4-MeC6H4 | iPr | 4h | 7 d | 65 |
| 9 | 1d, Bn | 2a, 4-MeC6H4 | iPr | 4i | 8 d | 64 |
| 10 | 1a, Me | 2a, 4-MeC6H4 | iPr | 3a | 3 h | 46d |
| 11 | 1a, Me | 2g, Cy | iPr | 4j | 1 d | e |
Reaction scale: 1 (0.7 mmol, 1.0 equiv), 2 (0.7 mmol, 1.0 equiv), KOAc (0.7 mmol, 1.0 equiv), and acetic acid (0.4 μL, 1 mol %) in CH3CN (3 mL) at RT.
After silica gel column chromatography.
10–20% of 1 and 2 was recovered; prolonged reaction led to a complex mixture.
Short reaction time allows isolation of the product before aromatization in this case.
Complex mixture.