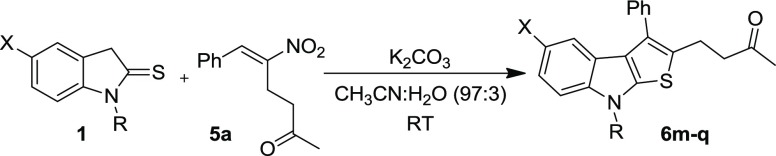
Table 5. Synthesis of Thienoindoles (6m–q) from RC Adduct 5a and Differently Substituted Indoline-2-thiones 1a.
| entry | 1, R | X | 6 | time (h) | % yieldb |
|---|---|---|---|---|---|
| 1 | 1b, Et | H | 6m | 12 | 87 |
| 2 | 1c, nPr | H | 6n | 12 | 55 |
| 3 | 1d, Bn | H | 6o | 13 | 94 |
| 4 | 1e, Me | Cl | 6p | 5 | 44 |
| 5 | 1f, H | H | 6q | 7 | 44 |
Reaction scale: 1a (0.75 mmol, 1.0 equiv), 5 (0.75 mmol, 1.0 equiv), K2CO3 (0.75 mmol, 1.0 equiv) in CH3CN, and H2O (97:3 v/v, 3 mL) at RT.
After silica-gel column chromatography.