Figure 5.
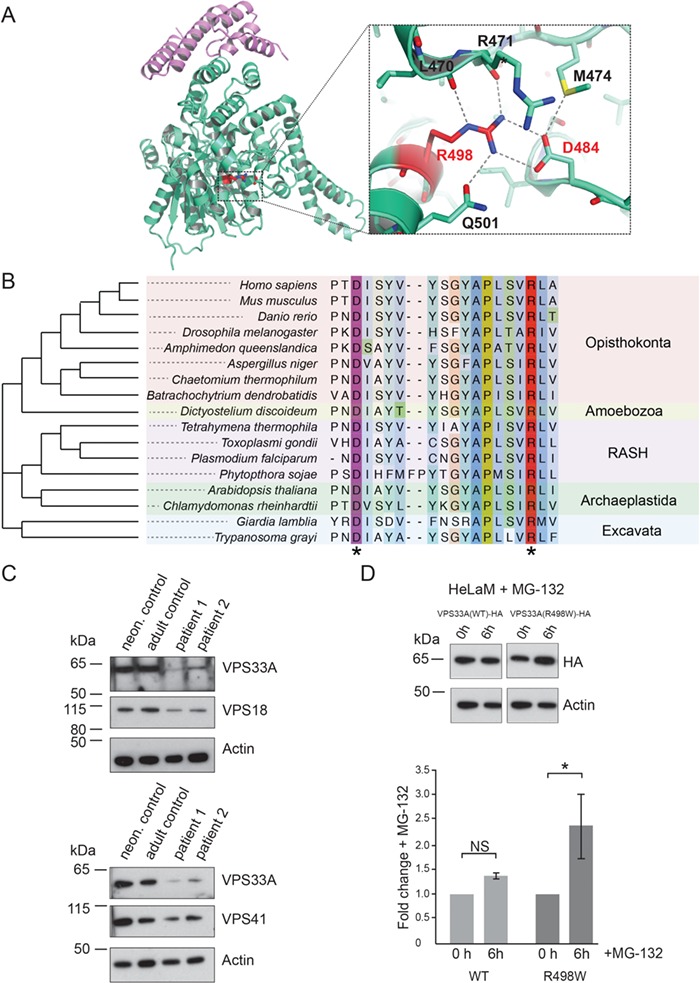
The R498W mutation causes instability of the VPS33A protein. (A) Cartoon representation of the crystal structure of human VPS33A (green) in complex with VPS16 (residues 642–736, purple; PDB ID 4xb9; 15). Arginine R498 (highlighted red) forms a salt bridge with aspartate D484 and participates in an extensive network of hydrogen bonds (blow up). (B) Phylogenetic tree and alignment of VPS33A show conservation of D484 (purple asterisk) and R498 (red asterisk) among species across all eukaryotic supergroups. RASH: Rhizaria, Alveolata, Stramenopila, Haptophyta. (C) Immunoblot analysis showing reduced abundance of VPS33A protein in patient fibroblasts (P1, P2) compared to neonatal (neon) and adult control fibroblasts. In patient fibroblasts, reduced protein levels were also observed for the HOPS/CORVET component VPS18 (upper panel) and the HOPS component VPS41 (lower panel). Actin was used as a loading control. (D) HelaM cells stably expressing VPS33AWT-HA or VPS33AR498W-HA were incubated with 10 μM MG-132 (proteasome inhibitor) for 6 h at 37°C in normal cell culture media (RPMI supplemented with 10% (v/v) FBS, 2 mM glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin) and cell lysates analysed by immunoblotting with anti-HA or anti-actin (loading control) antibodies. Immunoblots from four separate experiments (representative example in upper panel) were scanned and the densities of VPS33A bands quantified and normalized relative to actin. Fold changes in VPS33A concentration are shown relative to no incubation with MG-132 (lower panel). Error bars show SEM. NS, not significant; *, P < 0.05.
