Figure 1.
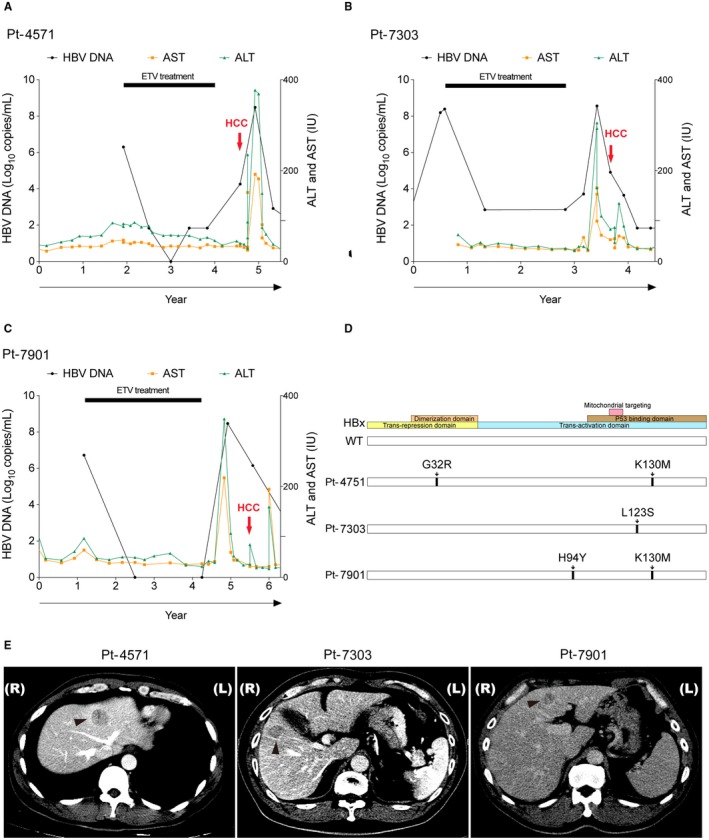
Clinical courses of 3 entecavir‐experienced patients with HCC. (A‐C) The clinical courses of Pt‐4571, a 44‐year‐old male (A), Pt‐7303, a 61‐year‐old male (B), and Pt‐7901, a 54‐year‐old male (C), were depicted. They were all negative for hepatitis B e antigen. At the time of HCC diagnosis, HBV‐DNA was 4.3, 4.8, and 6.2 log10/mL, and alpha‐fetoprotein was 51.3, 7.9, and 8.5 ng/mL, respectively. Black circles, HBV‐DNA; orange squares, aspartate aminotransferase; green triangles, alanine aminotransferase; red arrows, time of HCC diagnosis; horizonal bars, entecavir treatment period; horizontal axis, years of follow‐ups. These patients did not receive prior antiviral treatments. All patients were negative for anti‐HCV antibody, not alcoholic, not diabetics, and without family history of HCC. All had liver cirrhosis due to chronic hepatitis B. After treatments, all 3 patients achieved complete remission with no HCC recurrence up to 5 years of follow‐ups. (D) The positions of amino acid substitution mutations on HBx open reading frames. (E) Dynamic computed tomography for the 3 patients with HCC showing wash‐out of contrast medium. All three tumors were hyperdense in arterial phase but became hypodense in early venous phase (Pt‐4571 and Pt‐7303) or in delayed phase (Pt‐7901). All three HCCs were histologically proved. Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; ETV, entecavir.
