Figure 2.
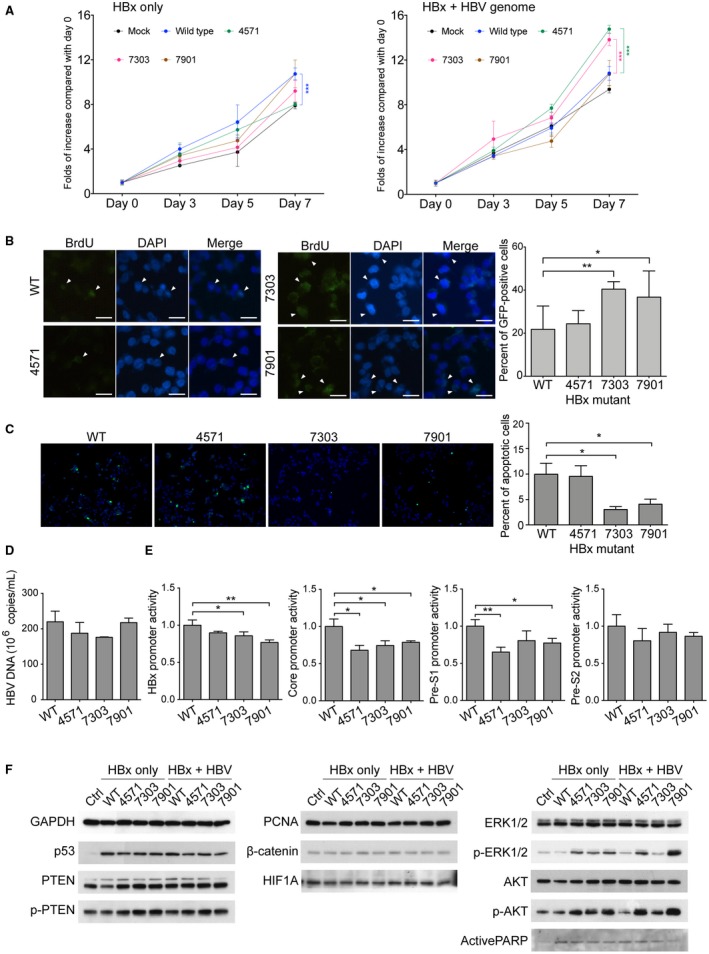
Antiviral treatment‐related HBx mutants had greater cell proliferation‐enhancing and apoptosis‐suppressing abilities, compared with the wild‐type HBx, in the presence of replication‐competent HBV genome. (A) 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide assays for J7 cells expressing the wild‐type or mutant HBx in the absence (left) or the presence (right) of replication‐competent HBV genome. Black circle, mock control; blue circle, wild‐type HBx; green circle, Pt‐4571‐derived HBx mutant; red circle, Pt‐7303‐derived HBx mutant; brown circle, Pt‐7901‐derived HBx mutant. ***, P < 0.001 (pared t test). (B) Bromodeoxyuridine incorporation assay in the presence of HBV genome. *, P < 0.05; **, P < 0.005. (C) Terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick‐end labeling assays for J7 cells expressing the wild‐type or mutant HBx in the presence of HBV genome. *, P < 0.05. (D) HBV‐DNA levels in the mediums of J7 cells expressing the wild‐type or mutant HBx in the presence of HBV genome. (E) The activities of HBx, Core, Pre‐S1 and Pre‐S2 promoters were assayed by luciferase reporter system following transient expression of the wild‐type or mutant HBx. (F) The levels of key molecules in different signaling pathways were examined by western blot. Abbreviations: Ctrl, control; DAPI, 4´,6‐diamidino‐2‐phenylindole; GAPDH, glyceraldehyde 3‐phosphate dehydrogenase; GFP, green fluorescent protein; HIF1A, hypoxia inducible factor 1 alpha subunit; PARP, poly(adenosine diphosphate ribose) polymerase; PTEN, phosphatase and tensin homolog; WT, wild type.
