Abstract
A mild base-catalyzed protocol for the synthesis of substituted nitroalkane derivatives has been developed under continuous flow using a microreaction technique. This transformation basically involves the 1,6-conjugate addition of nitroalkanes to p-quinone methides, leading to the substituted nitroalkanes in good to excellent yields.
Introduction
Over the past several decades, the chemistry of nitroalkanes has been recognized as one of the imperative research areas in synthetic chemistry.1 Nitroalkanes serve as a valuable synthon in the synthesis of natural products,2 biologically active unnatural active pharmaceutical ingredients (APIs),3 and other useful compounds, such as carbonyl compounds, carbohydrates, heterocycles, peptides, and so forth.4 Although there are several different methods available for the synthesis of nitroalkanes, the most popular one is the introduction of a nitro group through the displacement reaction between the alkyl halides and suitable inorganic or organic nitrite sources.5 Subsequently, other substituted nitroalkanes could be easily accessed from simple nitroalkanes through the Henry or nitro–aldol reactions,6 alkylation/arylation reactions, and so forth.7 Moreover, the 1,4-conjugate addition of nitroalkanes to enones,8 especially the enantioselective version, to access other functionalized nitroalkanes in an enantiomerically pure form, has been well-explored using either chiral organocatalysts9 or transition-metal catalysts.10 In addition, the 1,6-conjugate addition of nitroalkanes to the linear dienone system has also been investigated using appropriate catalytic systems.11 However, to the best of our knowledge, the 1,6-conjugate addition of nitroalkanes to p-quinone methides (p-QMs) has not been reported so far, although several reports,12 including ours,13 are available for the vinylogous Michael addition of other active methylene compounds to p-QMs. Consequently, while working on the nucleophilic addition reactions of p-QMs,14 we have developed a base-catalyzed method for the synthesis of highly substituted nitroalkane derivatives through the 1,6-conjugate addition of nitroalkanes to p-QMs (Scheme 1), and the results are disclosed herein.
Scheme 1. 1,6-Conjugate Addition of Nitroalkanes to p-QMs.
As the microreaction technique is emerging as a better alternative to the batch reaction technique because of its operational simplicity and higher efficiency,15 we have decided to develop this methodology under continuous flow using a microreactor. This particular technique has found many applications in synthetic organic chemistry, especially in methods development,16 synthesis of natural products and APIs,17 and asymmetric synthesis.18 In the recent past, we have also utilized this technique to access diarylalkanes and diarylmethyl thioethers through the 1,6-conjugate addition of dialkylzinc reagents and thiols, respectively, to p-QMs under continuous flow.19
Results and Discussion
The optimization experiments were performed with p-QM 1a and 2-nitropropane (2a) under continuous flow conditions using a commercial glass microreactor having a total volume of 100 μL. In all the experiments, a mixture of 1a and 2a was dissolved in 1 mL of solvent and injected into the microreactor through a syringe. Parallelly, a solution of a base in 1 mL of solvent was introduced into the microreactor through another syringe.
The results of the optimization studies are summarized in Table 1. Initially, a couple of experiments were carried out using toluene as the solvent at room temperature (rt) and at 80 °C (residence time = 2.5 min). However, in both the experiments, the required product 3a was not observed (entries 1 and 2). To our delight, when the reaction was performed in dimethyl sulfoxide (DMSO) with the residence time of 2.5 min, 3a was isolated in 58% yield (entry 3). However, in this case, we observed that 1a was slowly crystallizing in the syringe over a period of time, and because of this some blockage was observed in the microchannels. Hence, we decided to explore the combination of a polar and a nonpolar solvent for further studies. Subsequently, when the reaction was carried out in DMSO/toluene (98:2) mixture, the yield of 3a was increased to 74% at rt (entry 4). The yield of 3a was further improved to 78% by increasing the temperature to 80 °C under the flow rate of 40 μL/min (entry 5). At this point, we believed that the yield of 3a would be improved to a great extent if the residence time of the reaction mixture in the microchannels is increased. Accordingly, we conducted another experiment by increasing the residence time to 10 min, and, as expected, 3a was obtained in 91% yield (entry 6). Unfortunately, other bases such as 4-DMAP and 1,4-diazabicyclo-[2.2.2]octane (DABCO) failed to catalyze the reaction (entries 7 and 8). DBN (1,5-diazabicyclo-[4.3.0]non-5-ene) was found to be effective for this transformation, but the product 3a was obtained only in 60% yield (entry 9). Next, to find the best solvent system for this transformation, a few optimization experiments were performed in other solvents (entries 10–13). In the case of MeCN and dimethylformamide (DMF), 3a was isolated in 67 and 65% yields, respectively (entries 10 and 12), but the reaction did not proceed in other solvents such as 1,4-dioxane and tetrahydrofuran (THF; entries 11 and 13). Lowering the catalyst loading (5–15 mol %) affected the yield of 3a considerably (entries 14–16). Another experiment was carried out without the base catalyst and, in this case, no reaction was observed (entry 17). This experiment obviously signifies that a base catalyst is required for this transformation.
Table 1. Optimization Studiesa.
| entry | base | solvent | flow rate 1a and 2a + base in solvent [μL min–1] | residence time [min] | temp [°C] | yield of 3a [%] |
|---|---|---|---|---|---|---|
| 1 | DBU | PhMe | 20 + 20 | 2.5 | rt | nd |
| 2 | DBU | PhMe | 20 + 20 | 2.5 | 80 | nd |
| 3 | DBU | DMSO | 20 + 20 | 2.5 | rt | 58 |
| 4 | DBU | DMSO/PhMe (98:2) | 20 + 20 | 2.5 | rt | 74 |
| 5 | DBU | DMSO/PhMe (98:2) | 20 + 20 | 2.5 | 80 | 78 |
| 6 | DBU | DMSO/PhMe (98:2) | 5 + 5 | 10 | 80 | 91 |
| 7 | 4-DMAP | DMSO/PhMe (98:2) | 5 + 5 | 10 | 80 | nd |
| 8 | DABCO | DMSO/PhMe (98:2) | 5 + 5 | 10 | 80 | trace |
| 9 | DBN | DMSO/PhMe (98:2) | 5 + 5 | 10 | 80 | 60 |
| 10 | DBU | MeCN | 5 + 5 | 10 | 60 | 67 |
| 11 | DBU | 1,4-dioxane | 5 + 5 | 10 | 80 | trace |
| 12 | DBU | DMF | 5 + 5 | 10 | 80 | 65 |
| 13 | DBU | THF | 5 + 5 | 10 | 60 | nd |
| 14b | DBU | DMSO/PhMe (98:2) | 5 + 5 | 10 | 80 | 45 |
| 15c | DBU | DMSO/PhMe (98:2) | 5 + 5 | 10 | 80 | 53 |
| 16d | DBU | DMSO/PhMe (98:2) | 5 + 5 | 10 | 80 | 71 |
| 17 | DMSO/PhMe (98:2) | 5 + 5 | 10 | 80 | nd |
Reaction conditions: All the experiments were carried out with 0.12 mmol of 1a, 0.24 mmol of 2a, and 0.024 mmol of DBU in the solvent.
5 mol % of DBU was used.
10 mol % of DBU was used.
15 mol % of DBU was used;nd = not detected.
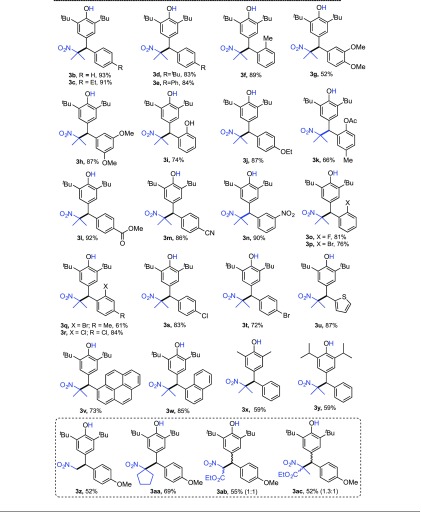
After finding the best reaction condition (entry 6, Table 1), the general applicability of this protocol was evaluated using a wide range of p-QMs and nitroalkanes (Table 2). Most of the p-QMs 1b–k (substituted with electron-rich arenes) underwent the 1,6-addition reaction with 2a and provided the corresponding 1,6-adducts (3b–k) in the range of 66–93% yields. Other p-QMs 1l–n (substituted with electron-poor arenes) also reacted with 2a efficiently and gave the respective products 3l–n in excellent yields (86–92%). The substrate scope was extended to haloarene-substituted (1o–t) and heteroaryl-substituted (1u) p-QMs and, in these cases, the 1,6-addition products 3o–u were obtained in the range of 61–84% yields.
Table 2. Substrate Scopea.
Reaction conditions: all the experiments were carried out in 40 mg scale of 1(b–y). Yields reported are isolated yields.
In the cases of p-QMs 1v and 1w (derived from fused aromatic aldehydes), the products 3v and 3w were isolated in 73 and 85% yields, respectively. Delightfully, the present protocol worked moderately well in the cases of other p-QMs 1x and 1y (derived from 2,6-dimethylphenol and propofol, respectively), and the products 3x and 3y were obtained in 59% yields. Other nitroalkanes such as nitromethane (2b) and 1-nitro-cyclopentane (2c) also reacted with 1a under standard conditions and gave the respective products 3z and 3aa in 52 and 69% yields, respectively. When ethyl nitroacetate was used as a nucleophile, the respective product 3ab was obtained in 55% yield as a mixture of diastereomers (dr = 1:1). Similarly, ethyl 2-nitropropionate provided the corresponding product 3ac in 52% yield and 1.3:1 diastereomeric ratio.
After evaluating the scope and limitations of this transformation using several p-QMs and nitroalkanes (Table 2), we wanted to compare the efficiency of the continuous flow process against the batch process in a large scale. In this regard, a gram-scale reaction was performed using 1a and 2a under continuous flow as well as batch processes (Scheme 2). As expected, in the case of the continuous flow reaction, the product 3a was obtained in 80% yield. When the same reaction was carried out at rt under batch conditions, 3a was obtained in 69% yield after 24 h and, in this case, complete consumption of 1a was not observed. However, when the batch reaction was carried out at 80 °C (standard conditions), 3a was obtained only in 54% yield. As the yield of 3a was substantially low at 80 °C, and also the complete conversion of 1a was not observed even after 3 days, we suspected that the retro-vinylogous Michael-type reaction was taking place in the case of the batch process. We believe that this could be the main reason for the lower yield of the product in the batch method. To confirm this, a batch reaction was carried out, in which the 1,6-adduct 3a was treated with 20 mol % 1,8-diazabicyclo-[5.4.0]undec-7-ene (DBU) in DMSO/toluene mixture at 80 °C and, as expected, the p-QM 1a was obtained in 69% yield (Scheme 2). Interestingly, when the same batch reaction was carried out at rt, 1a was observed only in trace amounts. The above-mentioned experiments led to the following conclusions. The 1,6-addition reaction between 1a and 2a is reversible only at a higher temperature (in this case, 80 °C). Therefore, one can conclude that, in the batch process, as the product 3a stays in the reaction flask for a longer period, the extent of retro-1,6-addition reaction is high. On the other hand, in the continuous flow method, though the reaction channels are kept at 80 °C, the reaction mixture does not stay in the microchannels for a long time. In fact, it is continuously removed from the microchannels through the outlet and collected in a flask that is kept at rt. This means the reaction mixture gets cooled to rt immediately after coming out of the microchannels. This clearly signifies that the retro-1,6-addition reaction is not possible in this case as this reaction does not take place at rt (Scheme 2). This is the primary reason for getting a higher yield of 3a in the continuous flow method when compared to the batch process. From this observation, one can also conclude that the continuous flow method is more advantageous than the batch process for this particular transformation.
Scheme 2. Batch vs Continuous Flow Processes and Control Experiments.
The enantioselective version of this transformation was attempted using a couple of chiral amine catalysts. A few reactions were carried out in the microreactor with 1d and 2a using 20 mol % of quinine (I) or quinidine (II) under different conditions, and the results are summarized in Scheme 3. When the reaction was carried out at rt using I or II, the product 3d was not formed in either case. However, when another reaction was performed with I or II at 80 °C, 3d was obtained in 51 and 33% yields, respectively. Unfortunately, in both the cases, adduct 3d was obtained as a racemic mixture (Scheme 3).
Scheme 3. Attempted Enantioselective Reactions with Chiral Bases under Continuous Flow.
Conclusions
In conclusion, an effective method for the synthesis of highly substituted nitroalkane derivatives has been developed under continuous flow conditions using the microreaction technology. This reaction proceeds through a base-catalyzed 1,6-conjugate addition of nitroalkanes to p-QMs, and the respective 1,6-adducts were obtained in good yields. The comparison between the continuous flow method and the batch process through control experiments revealed that the former method is more advantageous when compared to the latter process, as the retro-1,6-reaction was arrested in the former case and, as a result, the 1,6-adducts were obtained in good to excellent yields.
Experimental Section
General Methods
Continuous-flow reactions were performed using a FlowStart Evo B-401 instrument purchased from Future Chemistry Holding B.V. The microreactor was made up of borosilicate glass (channel width 600 μm; channel depth 500 μm), with an effective reaction volume of 100 μL. The microreactor setup has in-built syringe pumps and all the reactions were carried out without using a back pressure regulator. Most of the reagents and starting materials were purchased from commercial sources and used without further purification. All p-QMs were prepared by following the literature procedure.12a The melting points were recorded on an SMP20 melting point apparatus and are uncorrected. The 1H, 13C, and 19F spectra were recorded in CDCl3 (400, 100, and 376 MHz, respectively) on a Bruker FT-NMR spectrometer. The chemical shift (δ) values are reported in parts per million relative to tetramethylsilane, and the coupling constants (J) are reported in Hertz (Hz). High-resolution mass spectra were recorded on a Waters Q-TOF Premier-HAB213 spectrometer. The Fourier-transform infrared (FT-IR) spectra were recorded on a PerkinElmer FTIR spectrometer. Thin-layer chromatography was performed on Merck silica gel 60 F254 TLC pellets. Column chromatography was carried out through silica gel (100–200 mesh) using EtOAc/hexane as an eluent.
General Procedure for the 1,6-Conjugate Reaction of Nitroalkanes to p-Quinone Methides under Continuous Flow
p-QM (0.12 mmol, 1 equiv) and 2-nitroalkane (0.24 mmol, 2 equiv) were dissolved in 1 mL of DMSO/toluene (98:2) mixture and taken in a syringe. DBU (0.024 mmol, 20 mol %) was dissolved in 1 mL of DMSO/toluene (98:2) mixture and taken in another syringe. These two solutions were injected simultaneously through the microchannels at the flow rates of 5 μL/min each (residence time = 10 min). The temperature of the microchannels was maintained at 80 °C throughout the reaction. The reaction mixture was collected at the outlet and was quenched with water. It was extracted with diethyl ether (10 mL × 2). The organic layer was concentrated under reduced pressure, and the crude was then loaded on a silica gel column and purified using the hexane/EtOAc mixture as an eluent to provide the pure 1,6-adduct.
2,6-Di-tert-butyl-4-(1-(4-methoxyphenyl)-2-methyl-2-nitropropyl)phenol (3a)
The reaction was performed at 40 mg scale (0.123 mmol) of 1a; Rf = 0.3 (5% EtOAc in hexane); pale yellow solid (46.3 mg, 91% yield); mp 143–145 °C; 1H NMR (400 MHz, CDCl3): δ 7.29 (d, J = 8.3 Hz, 2H), 7.12 (s, 2H), 6.83 (d, J = 8.4 Hz, 2H), 5.12 (s, 1H), 4.62 (s, 1H), 3.77 (s, 3H), 1.64 (s, 3H), 1.63 (s, 3H), 1.41 (s, 18H); 13C NMR (100 MHz, CDCl3): δ 158.8, 153.0, 135.7, 131.5, 130.7, 129.5, 126.2, 114.0, 92.9, 60.0, 55.3, 34.5, 30.4, 25.4, 25.0; FT-IR (thin film, neat): 3421, 2958, 1263, 753 cm–1; HRMS (ESI) m/z: calcd for C25H35NO4 [M – H]−, 412.2488; found: 412.2470.
2,6-Di-tert-butyl-4-(2-methyl-2-nitro-1-phenylpropyl)phenol (3b)
The reaction was performed at 40 mg scale (0.135 mmol) of 1b; Rf = 0.4 (5% EtOAc in hexane); yellow solid (48.4 mg, 93% yield); mp 98–100 °C; 1H NMR (400 MHz, CDCl3): δ 7.38 (d, J = 7.7 Hz, 2H), 7.30 (t, J = 7.5 Hz, 2H), 7.26–7.22 (m, 1H), 7.14 (s, 2H), 5.14 (s, 1H), 4.67 (s, 1H), 1.66 (s, 6H), 1.42 (s, 18H); 13C NMR (100 MHz, CDCl3): δ 153.1, 139.5, 135.7, 129.7, 129.1, 128.6, 127.4, 126.3, 92.8, 60.7, 34.5, 30.4, 25.4, 25.2; FT-IR (thin film, neat): 3634, 2959, 1264, 750 cm–1; HRMS (ESI) m/z: calcd for C24H33NO3 [M – H]−, 382.2382; found, 382.2365.
2,6-Di-tert-butyl-4-(1-(4-ethylphenyl)-2-methyl-2-nitropropyl)phenol (3c)
The reaction was performed at 40 mg scale (0.124 mmol) of 1c; Rf = 0.4 (5% EtOAc in hexane); pale yellow gummy solid (46.5 mg, 91% yield); 1H NMR (400 MHz, CDCl3): δ 7.30 (d, J = 8.0 Hz, 2H), 7.15–7.12 (m, 4H), 5.12 (s, 1H), 4.63 (s, 1H), 2.61 (q, J = 7.6 Hz, 2H), 1.65 (s, 3H), 1.64 (s, 3H), 1.41 (s, 18H), 1.20 (t, J = 7.6 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 153.1, 143.3, 136.6, 135.6, 129.6, 129.3, 128.1, 126.3, 92.9, 60.5, 34.5, 30.4, 28.4, 25.4, 25.1, 15.5; FT-IR (thin film, neat): 3437, 2963, 1261, 752 cm–1; HRMS (ESI) m/z: calcd for C26H37NO3 [M – H]−, 410.2695; found, 410.2677.
2,6-Di-tert-butyl-4-(1-(4-(tert-butyl) phenyl)-2-methyl-2-nitropropyl)phenol (3d)
The reaction was performed at 40 mg scale (0.113 mmol) of 1d; Rf = 0.4 (5% EtOAc in hexane); pale yellow solid (41.3 mg, 83% yield); mp 134–136 °C; 1H NMR (400 MHz, CDCl3): δ 7.31 (s, 4H), 7.17 (s, 2H), 5.13 (s, 1H), 4.63 (s, 1H), 1.65 (s, 3H), 1.64 (s, 3H), 1.42 (s, 18H), 1.28 (s, 9H); 13C NMR (100 MHz, CDCl3): δ 153.1, 150.1, 136.2, 135.6, 129.3, 126.4, 125.5, 93.0, 60.5, 34.52, 34.50, 31.4, 30.4 (2C), 25.3, 25.1; FT-IR (thin film, neat): 3636, 2960, 1266, 752 cm–1; HRMS (ESI) m/z: calcd for C28H41NO3 [M – H]−,438.3008; found, 438.3026.
4-(1-([1,1′-Biphenyl]-4-yl)-2-methyl-2-nitropropyl)-2,6-di-tert-butylphenol (3e)
The reaction was performed at 40 mg scale (0.107 mmol) of 1e; Rf = 0.5 (5% EtOAc in hexane); pale yellow solid (41.4 mg, 84% yield); mp 148–150 °C; 1H NMR (400 MHz, CDCl3): δ 7.58–7.52 (m, 4H), 7.46–7.40 (m, 4H), 7.35–7.31 (m, 1H), 5.15 (s, 1H), 4.72 (s, 1H), 1.70 (s, 3H), 1.68 (s, 3H), 1.42 (s, 18H); 13C NMR (100 MHz, CDCl3): δ 153.2, 140.6, 140.1, 138.5, 135.8, 130.1, 129.1, 128.9, 127.5, 127.3, 127.1, 126.3, 92.8, 60.5, 34.5, 30.4, 25.5, 25.1; FT-IR (thin film, neat): 3426, 2987, 1266, 751 cm–1; HRMS (ESI) m/z: calcd for C30H34NO3 [M – Na]−, 458.2695; found, 458.2676.
2,6-Di-tert-butyl-4-(2-methyl-2-nitro-1-(o-tolyl)propyl)phenol (3f)
The reaction was performed at 40 mg scale (0.129 mmol) of 1f; Rf = 0.4 (5% EtOAc in hexane); pale yellow gummy solid (45.9 mg, 89% yield); mp 136–138 °C; 1H NMR (400 MHz, CDCl3): δ 7.54 (d, J = 7.8 Hz, 1H), 7.20 (td, J = 6.4, 2.6 Hz, 1H), 7.16–7.10 (m, 2H), 7.04 (s, 2H), 5.11 (s, 1H), 5.03 (s, 1H), 2.38 (s, 3H), 1.73 (s, 3H), 1.66 (s, 3H), 1.38 (s, 18H); 13C NMR (100 MHz, CDCl3): δ 153.0, 138.4, 137.4, 135.5, 131.4, 128.5, 127.1, 127.0, 126.7, 126.0, 93.1, 54.4, 34.4, 30.4, 26.0, 25.6, 21.0; FT-IR (thin film, neat): 3632, 2961, 1267, 754 cm–1; HRMS (ESI) m/z: calcd for C25H35NO3 [M – H]−, 396.2539; found, 396.2556.
2,6-Di-tert-butyl-4-(1-(3,4-dimethoxyphenyl)-2-methyl-2-nitropropyl)phenol (3g)
The reaction was performed at 40 mg scale (0.112 mmol) of 1g; Rf = 0.1 (5% EtOAc in hexane); yellow gummy solid (25.6 mg, 52% yield); 1H NMR (400 MHz, CDCl3): δ 7.15 (s, 2H), 6.94–6.89 (m, 2H), 6.80 (d, J = 8.3 Hz, 1H), 5.14 (s, 1H), 4.60 (s, 1H), 3.87 (s, 3H), 3.85 (s, 3H), 1.65 (s, 3H), 1.63 (s, 3H), 1.41 (s, 18H); 13C NMR (100 MHz, CDCl3): δ 153.1, 148.6, 148.3, 135.7, 131.8, 129.2, 126.2, 121.9, 113.1, 111.1, 93.0, 60.3, 55.94, 55.92, 34.5, 30.4, 25.5, 25.0; FT-IR (thin film, neat): 3435, 2959, 1266, 751 cm–1; HRMS (ESI) m/z: calcd for C26H37NO3 [M – H]−, 442.2593; found, 442.2573.
2,6-Di-tert-butyl-4-(1-(3,5-dimethoxyphenyl)-2-methyl-2-nitropropyl)phenol (3h)
The reaction was performed at 40 mg scale (0.112 mmol) of 1h; Rf = 0.3 (5% EtOAc in hexane); pale yellow solid (43.5 mg, 87% yield); mp 134–136 °C; 1H NMR (400 MHz, CDCl3): δ 7.14 (s, 2H), 6.54 (d, J = 2.1 Hz, 2H), 6.35 (t, J = 2.0 Hz, 1H), 5.15 (s, 1H), 4.60 (s, 1H), 3.77 (s, 6H), 1.67 (s, 3H), 1.65 (s, 3H), 1.41 (s, 18H); 13C NMR (100 MHz, CDCl3): δ 160.7, 153.2, 141.5, 135.6, 128.8, 126.3, 108.1, 99.0, 92.8, 60.8, 55.4, 34.5, 30.4, 25.35, 25.30; FT-IR (thin film, neat): 3626, 2958, 1265, 750 cm–1; HRMS (ESI) m/z: calcd for C26H37NO3 [M – H]−, 442.2593; found, 442.2573.
2,6-Di-tert-butyl-4-(1-(2-hydroxyphenyl)-2-methyl-2-nitropropyl)phenol (3i)
The reaction was performed at 40 mg scale (0.128 mmol) of 1i; Rf = 0.1 (5% EtOAc in hexane); pale yellow gummy solid (39.2 mg, 76% yield); 1H NMR (400 MHz, CDCl3): δ 7.46 (dd, J = 7.8, 1.4 Hz, 1H), 7.12–7.09 (m, 3H), 6.94–6.90 (m, 1H), 6.74 (dd, J = 8.0, 0.92 Hz, 1H), 5.27 (s, 1H), 5.14 (s, 1H), 5.01 (brs, 1H), 1.71 (s, 3H), 1.68 (s, 3H), 1.39 (s, 18H); 13C NMR (100 MHz, CDCl3): δ 153.7, 153.1, 135.7, 129.5, 129.0, 128.3, 126.6, 126.5, 120.9, 116.5, 92.5, 51.0, 34.5, 30.4, 25.9, 25.4; FT-IR (thin film, neat): 3632, 3448, 2959, 1266, 752 cm–1; HRMS (ESI) m/z: calcd for C24H33NO4 [M – H]−, 398.2331; found, 398.2343.
2,6-Di-tert-butyl-4-(1-(4-ethoxyphenyl)-2-methyl-2-nitropropyl)phenol (3j)
The reaction was performed at 40 mg scale (0.118 mmol) of 1j; Rf = 0.5 (5% EtOAc in hexane); pale yellow solid (44.1 mg, 87% yield); mp 122–124 °C; 1H NMR (400 MHz, CDCl3): δ 7.27 (d, J = 8.9 Hz, 2H), 7.12 (s, 2H), 6.82 (d, J = 8.5 Hz, 2H), 5.12 (s, 1H), 4.61 (s, 1H), 4.01 (q, J = 7.0 Hz, 2H), 1.62 (s, 6H), 1.40 (s, 18H), 1.38 (t, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 158.2, 153.0, 135.6, 131.3, 130.7, 129.5, 126.2, 114.5, 93.0, 63.5, 60.0, 34.5, 30.4, 25.3, 25.1, 15.0; FT-IR (KBr): 3444, 2958, 1260, 753 cm–1; HRMS (ESI) m/z: calcd for C26H37NO4 [M + Na]+, 450.2620; found, 450.2604.
2-(1-(3,5-Di-tert-butyl-4-hydroxyphenyl)-2-methyl-2-nitropropyl)-4-methylphenyl Acetate (3k)
The reaction was performed at 40 mg scale (0.109 mmol) of 1k; Rf = 0.2 (5% EtOAc in hexane); pale yellow solid (32.7 mg, 66% yield); mp 128–130 °C; 1H NMR (400 MHz, CDCl3): δ 7.29 (d, J = 1.5 Hz, 1H), 7.07 (dd, J = 8.2, 1.5 Hz, 2H), 6.97 (s, 2H), 6.94 (d, J = 8.2 Hz, 1H), 5.11 (s, 1H), 4.99 (s, 1H), 2.34 (s, 3H), 2.27 (s, 3H), 1.72 (s, 3H), 1.61 (s, 3H), 1.37 (s, 18H); 13C NMR (100 MHz, CDCl3): δ 169.3, 153.0, 147.0, 135.5, 135.4, 131.6, 129.9, 128.8, 128.7, 126.2, 123.0, 92.2, 51.5, 34.4, 30.3, 26.5, 24.8, 21.5, 21.1; FT-IR (thin film, neat): 3632, 2966, 1764, 1268, 754 cm–1; HRMS (ESI) m/z: calcd for C27H37NO5 [M – H]−, 454.2593; found, 454.2571.
Methyl 4-(1-(3,5-Di-tert-butyl-4-hydroxyphenyl)-2-methyl-2-nitropropyl)benzoate (3l)
The reaction was performed at 40 mg scale (0.113 mmol) of 1l; Rf = 0.2 (5% EtOAc in hexane); pale yellow solid (46.4 mg, 92% yield); mp 145–147 °C; 1H NMR (400 MHz, CDCl3): δ 7.97 (d, J = 8.1 Hz, 2H), 7.44 (d, J = 8.2 Hz, 2H), 7.10 (s, 2H), 5.18 (s, 1H), 4.75 (s, 1H), 3.90 (s, 3H), 1.66 (s, 6H), 1.40 (s, 18H); 13C NMR (100 MHz, CDCl3): δ 166.9, 153.3, 144.7, 135.9, 129.9, 129.7, 129.2, 128.4, 126.3, 92.4, 60.5, 52.3, 34.5, 30.4, 25.8, 24.9; FT-IR (thin film, neat): 3633, 2957, 1723, 1281, 755 cm–1; HRMS (ESI) m/z: calcd for C26H35NO5 [M – H]−, 440.2437; found, 440.2427.
4-(1-(3,5-Di-tert-butyl-4-hydroxyphenyl)-2-methyl-2-nitropropyl)benzonitrile (3m)
The reaction was performed at 40 mg scale (0.125 mmol) of 1m; Rf = 0.1 (5% EtOAc in hexane); pale yellow solid (44.1 mg, 86% yield); mp 140–142 °C; 1H NMR (400 MHz, CDCl3): δ 7.59 (d, J = 8.1 Hz, 2H), 7.45 (d, J = 8.2 Hz, 2H), 7.06 (s, 2H), 5.22 (s, 1H), 4.72 (s, 1H), 1.67 (s, 3H), 1.64 (s, 3H), 1.41 (s, 18H); 13C NMR (100 MHz, CDCl3): δ 153.5, 145.0, 136.2, 132.4, 130.3, 127.9, 126.3, 118.7, 111.3, 92.1, 60.5, 34.5, 30.4, 26.4, 24.4; FT-IR (KBr): 3630, 2959, 2230, 1264, 752 cm–1; HRMS (ESI) m/z: calcd for C25H32N2O3 [M – H]−, 407.2335; found, 407.2326.
2,6-Di-tert-butyl-4-(2-methyl-2-nitro-1-(3-nitrophenyl)propyl)phenol (3n)
The reaction was performed at 40 mg scale (0.117 mmol) of 1n; Rf = 0.2 (5% EtOAc in hexane); pale yellow solid (45.2 mg, 90% yield); mp 130–132 °C; 1H NMR (400 MHz, CDCl3): δ 8.33 (t, J = 2.0 Hz, 1H), 8.14–8.11 (m, 1H), 7.68 (d, J = 7.8 Hz, 1H), 7.49 (t, J = 8.0 Hz, 1H), 7.14 (s, 2H), 5.23 (s, 1H), 4.77 (s, 1H), 1.71 (s, 3H), 1.66 (s, 3H), 1.42 (s, 18H); 13C NMR (100 MHz, CDCl3): δ 153.6, 148.3, 141.5, 136.2, 135.7, 129.6, 127.7, 126.3, 124.4, 122.5, 92.2, 60.1, 34.5, 30.4, 26.4, 24.2; FT-IR (thin film, neat): 3629, 2958, 1240, 736 cm–1; HRMS (ESI) m/z: calcd for C24H32N2O5 [M – H]−, 427.2233; found, 427.2214.
2,6-Di-tert-butyl-4-(1-(2-fluorophenyl)-2-methyl-2-nitropropyl)phenol (3o)
The reaction was performed at 40 mg scale (0.128 mmol) of 1o; Rf = 0.4 (5% EtOAc in hexane); yellow solid (41.7 mg, 81% yield); mp 103–105 °C; 1H NMR (400 MHz, CDCl3): δ 7.52 (td, J = 7.7, 1.4 Hz, 1H), 7.24–7.20 (m, 1H), 7.12 (d, J = 7.8 Hz, 1H), 7.11 (s, 2H), 7.05 (t, J = 10.2 Hz, 1H), 5.15 (s, 1H), 5.11 (s, 1H), 1.70 (s, 3H), 1.65 (s, 3H), 1.40 (s, 18H); 13C NMR (100 MHz, CDCl3): δ 161.0 (d, JC–F = 244.5 Hz), 153.2, 135.7, 130.1 (d, JC–F = 2.9 Hz), 129.0 (d, JC–F = 8.7 Hz), 128.4, 127.0 (d, JC–F = 13.3 Hz), 126.4, 124.1 (d, JC–F = 3.6 Hz), 116.0 (d, JC–F = 24 Hz), 92.1, 51.2 (d, JC–F = 3.7 Hz), 34.5, 30.4, 26.1, 25.1; 19F NMR (376 MHz, CDCl3): δ −114.50; FT-IR (KBr): 3436, 2963, 1264, 751 cm–1; HRMS (ESI) m/z: calcd for C24H32FNO3 [M – H]−, 400.2288; found, 400.2270.
4-(1-(2-Bromophenyl)-2-methyl-2-nitropropyl)-2,6-di-tert-butylphenol (3p)
The reaction was performed at 40 mg scale (0.107 mmol) of 1p; Rf = 0.4 (5% EtOAc in hexane); pale yellow solid (37.8 mg, 76% yield); mp 156–158 °C; 1H NMR (400 MHz, CDCl3): δ 7.62–7.58 (m, 2H), 7.30 (t, J = 7.6 Hz, 1H), 7.11–7.09 (m, 1H), 7.07 (s, 2H), 5.41 (s, 1H), 5.14 (s, 1H), 1.74 (s, 3H), 1.67 (s, 3H), 1.39 (s, 18H); 13C NMR (100 MHz, CDCl3): δ 153.2, 139.3, 135.6, 133.8, 129.1, 128.7, 128.1, 127.5, 127.0, 126.6, 92.3, 57.4, 34.5, 30.4, 26.2, 25.4; FT-IR (KBr): 3633, 2957, 1240, 754 cm–1; HRMS (ESI) m/z: calcd for C24H32 BrNO3 [M – H]−, 460.1487; found, 460.1465.
4-(1-(2-Bromo-4-methylphenyl)-2-methyl-2-nitropropyl)-2,6-di-tert-butylphenol (3q)
The reaction was performed at 40 mg scale (0.103 mmol) of 1q; Rf = 0.5 (5% EtOAc in hexane); pale yellow solid (29.8 mg, 61% yield); mp 141–143 °C; 1H NMR (400 MHz, CDCl3): δ 7.48 (d, J = 8.1 Hz, 1H), 7.42 (d, J = 1.0 Hz, 1H), 7.10 (dd, J = 8.2, 1.2 Hz, 1H), 7.07 (s, 2H), 5.34 (s, 1H), 5.13 (s, 1H), 2.28 (s, 3H), 1.73 (s, 3H), 1.66 (s, 3H), 1.39 (s, 18H); 13C NMR (100 MHz, CDCl3): δ 153.1, 138.8, 136.1, 135.5, 134.2, 128.8, 128.4, 128.3, 126.8, 126.5, 92.3, 57.1, 34.5, 30.4, 26.2, 25.3, 20.7; FT-IR (thin film, neat): 3425, 2958, 1266, 751 cm–1; HRMS (ESI) m/z: calcd for C25H34BrNO3 [M – H]−, 474.1644; found, 474.1624.
2,6-Di-tert-butyl-4-(1-(2,4-dichlorophenyl)-2-methyl-2-nitropropyl)phenol (3r)
The reaction was performed at 40 mg scale (0.110 mmol) of 1r; Rf = 0.4 (5% EtOAc in hexane); pale yellow gummy solid (42.1 mg, 84% yield); 1H NMR (400 MHz, CDCl3): δ 7.53 (d, J = 8.6 Hz, 1H), 7.41 (d, J = 2.2 Hz, 1H), 7.24 (dd, J = 8.6, 2.3 Hz, 1H), 7.02 (s, 2H), 5.32 (s, 1H), 5.17 (s, 1H), 1.72 (s, 3H), 1.66 (s, 3H), 1.39 (s, 18H); 13C NMR (100 MHz, CDCl3): δ 153.3, 136.4, 136.2, 135.8, 133.5, 130.1, 129.9, 127.7, 127.2, 126.4, 91.8, 54.2, 34.5, 30.4, 26.0, 25.8; FT-IR (thin film, neat): 3632, 2961, 1266, 739 cm–1; HRMS (ESI) m/z: calcd for C24H31Cl2NO3 [M – H]−, 450.1603; found, 450.1584.
2,6-Di-tert-butyl-4-(1-(4-chlorophenyl)-2-methyl-2-nitropropyl)phenol (3s)
The reaction was performed at 40 mg scale (0.121 mmol) of 1s; Rf = 0.5 (5% EtOAc in hexane); pale yellow solid (42.1 mg, 83% yield); mp 134–136 °C; 1H NMR (400 MHz, CDCl3): δ 7.31–7.28 (m, 4H), 7.10 (s, 2H), 5.17 (s, 1H), 4.65 (s, 1H), 1.65 (s, 3H), 1.63 (s, 3H), 1.41 (s, 18H); 13C NMR (100 MHz, CDCl3): δ 153.2, 138.0, 135.9, 133.3, 130.9, 128.8, 128.7, 126.2, 92.5, 60.0, 34.5, 30.4, 25.8, 24.8; FT-IR (KBr): 3425, 2992, 1266, 752 cm–1; HRMS (ESI) m/z: calcd for C24H32ClNO3 [M – H]−, 416.1992; found, 416.1973.
4-(1-(4-Bromophenyl)-2-methyl-2-nitropropyl)-2,6-di-tert-butylphenol (3t)
The reaction was performed at 40 mg scale (0.107 mmol) of 1t; Rf = 0.5 (5% EtOAc in hexane); pale yellow solid (35.6 mg, 72% yield); mp 132–134 °C;1H NMR (400 MHz, CDCl3): δ 7.42 (d, J = 8.3 Hz, 2H), 7.23 (d, J = 8.3 Hz, 2H), 7.08 (s, 2H), 5.17 (s, 1H), 4.64 (s, 1H), 1.65 (s, 3H), 1.63 (s, 3H), 1.41 (s, 18H); 13C NMR (100 MHz, CDCl3): δ 153.3, 138.6, 135.9, 131.7, 131.3, 128.6, 126.2, 121.5, 92.4, 60.1, 34.5, 30.4, 25.8, 24.8; FT-IR (thin film, neat): 3629, 2961, 1266, 753 cm–1; HRMS (ESI) m/z: calcd for C24H32BrNO3 [M – H]−, 460.1487; found, 460.1466.
2,6-Di-tert-butyl-4-(2-methyl-2-nitro-1-(thiophen-2-yl)propyl)phenol (3u)
The reaction was performed at 40 mg scale (0.133 mmol) of 1u; Rf = 0.4 (5% EtOAc in hexane); pale yellow solid (45.3 mg, 87% yield); mp 135–137 °C; 1H NMR (400 MHz, CDCl3): δ 7.23 (s, 2H), 7.21 (d, J = 0.8 Hz, 1H), 6.98 (dd, J = 3.5, 0.8 Hz, 1H), 6.94 (dd, J = 5.1, 3.5 Hz, 1H), 5.19 (s, 1H), 5.05 (s, 1H), 1.68 (s, 3H), 1.60 (s, 3H), 1.44 (s, 18H); 13C NMR (100 MHz, CDCl3): δ 153.4, 141.1, 135.7, 128.1, 127.9, 126.6, 126.5, 125.2, 93.0, 56.3, 34.5, 30.4, 25.0, 23.9; FT-IR (thin film, neat): 3631, 2960, 1262, 753 cm–1; HRMS (ESI) m/z: calcd for C22H31NO3S [M – H]−, 388.1946; found, 388.1929.
2,6-Di-tert-butyl-4-(2-methyl-2-nitro-1-(pyren-1-yl)propyl)phenol (3v)
The reaction was performed at 40 mg scale (0.096 mmol) of 1v; Rf = 0.4 (5% EtOAc in hexane); orange solid (35.3 mg, 73% yield); mp 218–220 °C; 1H NMR (400 MHz, CDCl3): δ 8.60 (d, J = 9.5 Hz, 1H), 8.22–8.16 (m, 5H), 8.08–7.99 (m, 3H), 7.20 (s, 2H), 6.12 (s, 1H), 5.11 (s, 1H), 1.93 (s, 3H), 1.69 (s, 3H), 1.37 (s, 18H); 13C NMR (100 MHz, CDCl3): δ 152.9, 135.7, 133.8, 131.5, 130.7, 130.4, 130.1, 129.9, 128.3, 127.7, 127.5, 126.3, 126.2, 126.1, 125.6, 125.5, 125.2, 125.0, 124.6, 123.0, 93.3, 53.3, 34.5, 30.4, 27.2, 25.1; FT-IR (thin film, neat): 3433, 2963, 1266, 752 cm–1; HRMS (ESI) m/z: calcd for C34H37NO3 [M – H]−, 506.2695; found, 506.2679.
2,6-Di-tert-butyl-4-(2-methyl-1-(naphthalen-1-yl)-2-nitropropyl)phenol (3w)
The reaction was performed at 40 mg scale (0.116 mmol) of 1w; Rf = 0.4 (5% EtOAc in hexane); pale yellow solid (42.7 mg, 85% yield); mp 148–150 °C; 1H NMR (400 MHz, CDCl3): δ 8.29 (d, J = 8.6 Hz, 1H), 7.84 (dd, J = 7.9, 0.7 Hz, 1H), 7.77 (d, J = 8.3 Hz, 1H), 7.72 (d, J = 7.3 Hz, 1H), 7.56–7.51 (m, 1H), 7.47 (t, J = 7.9 Hz, 2H), 7.13 (s, 2H), 5.77 (s, 1H), 5.10 (s, 1H), 1.83 (s, 3H), 1.68 (s, 3H), 1.37 (s, 18H); 13C NMR (100 MHz, CDCl3): δ 153.0, 136.0, 135.6, 134.4, 132.7, 129.2, 129.1, 128.1, 126.6, 126.4, 125.7, 125.2, 125.0, 123.5, 93.1, 53.1, 34.5, 30.4, 26.3, 25.9; FT-IR (thin film, neat): 3631, 2959, 1238, 752 cm–1; HRMS (ESI) m/z: calcd for C28H35NO3 [M – H]−, 432.2539; found, 432.2521.
2,6-Dimethyl-4-(2-methyl-2-nitro-1-phenylpropyl)phenol (3x)
The reaction was performed at 40 mg scale (0.190 mmol) of 1x; Rf = 0.1 (5% EtOAc in hexane); pale yellow solid (33.6 mg, 59% yield); mp 93–95 °C; 1H NMR (400 MHz, CDCl3): δ 7.36–7.34 (m, 2H), 7.32–7.28 (m, 2H), 7.26–7.21 (m, 1H), 6.96 (s, 2H), 4.65 (s, 1H), 4.57 (s, 1H), 2.21 (s, 6H), 1.68 (s, 3H), 1.67 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 151.7, 139.4, 130.4, 129.9, 129.5, 128.7, 127.4, 123.1, 92.5, 60.0, 25.4, 25.3, 16.2; FT-IR (thin film, neat): 3469, 2968, 1268, 754 cm–1; HRMS (ESI) m/z: calcd for C18H21NO3 [M – H]−, 298.1443; found, 298.1456.
2,6-Diisopropyl-4-(2-methyl-2-nitro-1-phenylpropyl)phenol (3y)
The reaction was performed at 40 mg scale (0.150 mmol) of 1y; Rf = 0.2 (5% EtOAc in hexane); yellow gummy solid (31.4 mg, 59% yield); 1H NMR (400 MHz, CDCl3): δ 7.38–7.36 (m, 2H), 7.32–7.29 (m, 2H), 7.25–7.22 (m, 1H), 7.03 (s, 2H), 4.77 (s, 1H), 4.70 (s, 1H), 3.11 (sept, J = 6.8 Hz, 2H), 1.67 (s, 3H), 1.65 (s, 3H), 1.24 (d, J = 6.8 Hz, 6H), 1.23 (d, J = 6.8 Hz, 6H); 13C NMR (100 MHz, CDCl3): δ 149.4, 139.3, 133.6, 130.5, 129.6, 128.6, 127.4, 124.9, 92.7, 60.5, 27.3, 25.5, 25.1, 22.87, 22.85; FT-IR (thin film, neat): 3434, 2974, 1269, 754 cm–1; HRMS (ESI) m/z: calcd for C22H29NO3 [M – H]−, 354.2069; found, 354.2054.
2,6-Di-tert-butyl-4-(1-(4-methoxyphenyl)-2-nitroethyl)phenol (3z)
The reaction was performed at 40 mg scale (0.123 mmol) of 1a; Rf = 0.4 (5% EtOAc in hexane); yellow gummy solid (24.8 mg, 52% yield); 1H NMR (400 MHz, CDCl3): δ 7.18 (d, J = 8.7 Hz, 2H), 7.0 (s, 2H), 6.86 (d, J = 8.7 Hz, 2H), 5.15 (s, 1H), 4.95–4.85 (m, 2H), 4.76 (dd, J = 9.3, 7.2 Hz, 1H), 3.78 (s, 3H), 1.40 (s, 18H); 13C NMR (100 MHz, CDCl3): δ 158.9, 153.1, 136.3, 131.8, 130.1, 128.8, 124.3, 114.4, 80.2, 55.4, 48.6, 34.5, 30.3; FT-IR (thin film, neat): 3628, 2959, 1259, 754 cm–1; HRMS (ESI) m/z: calcd for C23H31NO4 [M – H]−, 384.2175; found, 384.2158.
2,6-Di-tert-butyl-4-((4-methoxyphenyl) (1-Nitrocyclopentyl)methyl)phenol (3aa)
The reaction was performed at 40 mg scale (0.123 mmol) of 1a; Rf = 0.3 (5% EtOAc in hexane); pale yellow solid (37.1 mg, 69% yield); mp 132–134 °C; 1H NMR (400 MHz, CDCl3): δ 7.26 (d, J = 8.7 Hz, 2H), 7.10 (s, 2H), 6.82 (d, J = 8.7 Hz, 2H), 5.11 (s, 1H), 4.83 (s, 1H), 3.77 (s, 3H), 2.74–2.70 (m, 2H), 2.11–2.04 (m, 2H), 1.64–1.58 (m, 2H), 1.53–1.50 (m, 2H), 1.40 (s, 18H); 13C NMR (100 MHz, CDCl3): δ 158.6, 152.9, 135.6, 132.1, 130.8, 130.0, 126.2, 113.9, 105.0, 57.5, 55.3, 35.19, 35.18, 34.5, 30.4, 23.4, 23.3; FT-IR (thin film, neat): 3630, 2960, 1259, 753 cm–1; HRMS (ESI) m/z: calcd for C25H35NO4 [M – H]−, 438.2644; found, 438.2664.
Ethyl 3-(3,5-Di-tert-butyl-4-hydroxyphenyl)-3-(4-methoxyphenyl)-2-nitropropanoate (3ab)
The reaction was performed at 0.123 mmol scale of 1a; Rf = 0.1 (5% EtOAc in hexane); pale yellow gummy solid (30.6 mg, 55% yield); the product was obtained as a 1:1 diastereomeric mixture. 1H NMR (400 MHz, CDCl3): δ 7.26 (d, J = 8.7 Hz, 2H), 7.21 (d, J = 8.7 Hz, 2H), 7.07 (s, 2H), 7.03 (s, 2H), 6.85 (d, J = 6.9 Hz, 2H), 6.83 (d, J = 6.9 Hz, 2H), 5.86 (d, J = 10.3 Hz, 1H), 5.83 (d, J = 10.3 Hz, 1H), 5.15 (s, 1H), 5.13 (s, 1H), 4.89 (d, J = 4.1 Hz, 1H), 4.86 (d, J = 4.1 Hz, 1H), 4.08–4.02 (m, 2H), 4.01–3.94 (m, 2H), 1.39 (s, 18H), 1.38 (s, 18H), 1.04 (t, J = 5.4 Hz, 3H), 0.94 (t, J = 5.4 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 163.7, 163.6, 159.1, 159.0, 153.4, 153.3, 136.3, 136.27, 129.4, 129.1, 128.5, 128.2, 124.9, 124.0, 114.5, 114.3, 91.98, 91.95, 63.0, 62.8, 55.37, 55.35, 52.0, 51.9, 34.49, 34.47, 30.33, 30.32, 13.8, 13.6; FT-IR (thin film, neat): 3627, 2960, 1748, 1565, 753 cm–1; HRMS (ESI) m/z: calcd for C26H35NO6 [M + Na]+, 480.2362; found, 480.2341.
Ethyl 3-(3,5-Di-tert-butyl-4-hydroxyphenyl)-3-(4-methoxyphenyl)-2-methyl-2-nitropropanoate (3ac)
The reaction was performed at 0.123 mmol scale of 1a; Rf = 0.1 (5% EtOAc in hexane); pale yellow gummy solid (30.2 mg, 52% yield); the product was obtained as a 1.3:1 diastereomeric mixture. Major diastereoisomer: 1H NMR (400 MHz, CDCl3): δ 7.32 (d, J = 2.1 Hz, 2H), 7.13 (s, 2H), 6.83 (d, J = 1.6 Hz, 2H), 5.25 (s, 1H), 5.14 (s, 1H), 4.03–3.98 (m, 2H), 3.76 (s, 3H), 1.93 (s, 3H), 1.40 (s, 18H), 0.99 (t, J = 7.1 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 166.6, 158.7, 153.07, 135.72, 131.1, 130.79, 126.4, 113.76, 97.6, 62.75, 55.32, 55.31, 34.5, 30.4, 20.8, 13.6; minor diastereoisomer: 1H NMR (400 MHz, CDCl3): δ 7.30 (d, J = 2.1 Hz, 2H), 7.14 (s, 2H), 6.80 (d, J = 1.7 Hz, 2H), 5.20 (s, 1H), 5.13 (s, 1H), 4.05 (q, J = 7.1 Hz, 2H), 3.77 (s, 3H), 1.94 (s, 3H), 1.39 (s, 18H), 1.07 (t, J = 7.2 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 166.7, 158.8, 153.09, 135.68, 131.0, 130.76, 126.7, 113.8, 97.5, 62.8, 55.36, 55.29, 34.5, 30.4, 21.0, 13.7; FT-IR (thin film, neat): 3620, 2965, 1740, 1561, 758 cm–1; HRMS (ESI) m/z: calcd for C27H37NO6 [M + Na]+, 472.2699; found, 494.2519.
Procedure for the Gram–Scale Reaction of 1a with 2a under Continuous-Flow Conditions
p-QMs 1a (1.0 g, 3.1 mmol) and 2-nitropropane 2a (0.56 mL, 6.2 mmol) were dissolved in 9 mL of DMSO/toluene (98:2) mixture and taken in a syringe. DBU (91 μL, 0.62 mmol) was dissolved in 9 mL of DMSO/toluene (90:10) mixture and taken in another syringe. These two solutions were injected simultaneously through the microchannels at the flow rates of 5 μL/min each (residence time = 10 min). The temperature of the microchannels was maintained at 80 °C throughout the reaction. The reaction mixture was collected at the outlet and was quenched with water. It was extracted with diethyl ether (50 mL × 3). The organic layer was concentrated under reduced pressure, and the crude was then loaded on a silica gel column and purified using the hexane/EtOAc mixture as an eluent to provide the pure 1,6-adduct 3a. The yield was 1.02 g (80%).
Procedure for the Gram–Scale Reaction of 1a with 2a under Conventional Batch Process
In a 100 mL round-bottom flask, p-QM 1a (1.0 g, 3.1 mmol), 2-nitropropane 2a (0.56 mL, 6.2 mmol), and DBU (90 μL, 0.61 mmol) were dissolved in 20 mL of DMSO/toluene (90:10) mixture, and the resulting mixture was stirred at 80 °C for 72 h. The mixture was cooled to rt and diluted with water. It was extracted with diethyl ether (50 mL × 3). The organic layer was concentrated under reduced pressure, and the crude was then loaded on a silica gel column and purified using the hexane/EtOAc mixture as an eluent to provide the pure 1,6-adduct 3a. The yield was 0.69 g (54%).
Procedure for the Retro-1,6-Conjugate Addition Reaction of 3a with DBU under Batch Process
In a 10 mL round-bottom flask, a mixture of 1,6-adduct 3a (40 mg, 0.096 mmol) and DBU (3 μL [stock solution], 0.019 mmol) was dissolved in 2 mL of DMSO/toluene (98:2) mixture, and the resulting mixture was stirred at 80 °C for 12 h. The mixture was cooled to rt and diluted with water. It was extracted with diethyl ether (10 mL × 3). The organic layer was concentrated under reduced pressure, and the crude was then loaded on a silica gel column and purified using the hexane/EtOAc mixture as an eluent to provide the pure 1,6-adduct 1a. The yield was 20.4 mg (65%).
Acknowledgments
The authors gratefully acknowledge DST-SERB (EMR/2015/001759) and IISER Mohali for the financial support. R.P. thanks DST, New Delhi for providing a research fellowship through the INSPIRE program. The NMR and HRMS facilities at IISER Mohali are gratefully acknowledged.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b01971.
1H and 13C NMR spectra of all new compounds, reaction mechanism, and actual photographs of the reaction setup (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Dabrowska-Urbańska H.; Katritzky A. R.; Urbanski T. Chemistry of Nitroalkanes – LXXXIII. Tetrahedron 1969, 25, 1617–1628. 10.1016/s0040-4020(01)82734-4. [DOI] [Google Scholar]; b Feuer H.; Nielson A. T.. Nitro Compounds: Recent Advances in Synthesis and Chemistry; VCH: New York, 1990. [Google Scholar]; c Ono N.The Nitro Group in Organic Synthesis; Wiley-VCH: New York, 2001. [Google Scholar]
- a For a review:Ballini R.; Petrini M.; Rosini G. Nitroalkanes as Central Reagents in the Synthesis of Spiroketals. Molecules 2008, 13, 319–330. and references cited therein 10.3390/molecules13020319. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Hoashi Y.; Yabuta T.; Yuan P.; Miyabe H.; Takemoto Y. Enantioselective tandem Michael reaction to nitroalkene catalyzed by bifunctional thiourea: total synthesis of (−)-epibatidine. Tetrahedron 2006, 62, 365–374. 10.1016/j.tet.2005.08.109. [DOI] [Google Scholar]; c Luo S.-P.; Guo L.-D.; Gao L.-H.; Li S.; Huang P.-Q. Toward the Total Synthesis of Haliclonin A: Construction of a Tricyclic Substructure. Chem.—Eur. J. 2013, 19, 87–91. 10.1002/chem.201203203. [DOI] [PubMed] [Google Scholar]; d Huang J.-Z.; Wu X.; Gong L.-Z. Enantioselective Organocatalytic Addition of Nitroalkanes to Oxindolylideneindolenines for the Construction of Chiral 3,3-Disubstituted Oxindoles. Adv. Synth. Catal. 2013, 355, 2531–2537. 10.1002/adsc.201300373. [DOI] [Google Scholar]; e Kudoh T.; Araki Y.; Miyoshi N.; Tanioka M.; Sakakura A. Diastereodivergent Henry Reaction for the Stereoselective Construction of Nitrogen-Containing Tetrasubstituted Carbons: Application to Total Synthesis of Manzacidins A and C. Asian J. Org. Chem. 2017, 6, 1760–1763. 10.1002/ajoc.201700568. [DOI] [Google Scholar]
- For selected examples:; a Roy A.; Reddy L. A.; Dwivedi N.; Naram J.; Swapna R.; Malakondaiah G. C.; Ravikumar M.; Bhalerao D.; Pratap T. B.; Reddy P. P.; Bhattacharya A.; Bandichhor R. Diastereoselective Synthesis of a Core Fragment of Ritonavir and Lopinavir. Tetrahedron Lett. 2011, 52, 6968–6970. 10.1016/j.tetlet.2011.10.087. [DOI] [Google Scholar]; b Rossi S.; Benaglia M.; Porta R.; Cotarca L.; Maragni P.; Verzini M. A Stereoselective Catalytic Nitroaldol Reaction as the Key Step in a Strategy for the Synthesis of the Renin Inhibitor Aliskiren. Eur. J. Org. Chem. 2015, 2531–2537. 10.1002/ejoc.201403659. [DOI] [Google Scholar]
- For selected examples:; a Ballini R.; Petrini M. The Nitro to Carbonyl Conversion (Nef Reaction): New Perspectives for a Classical Transformation. Adv. Synth. Catal. 2015, 357, 2371–2402. 10.1002/adsc.201500008. [DOI] [Google Scholar]; b Soengas R. G.; Estévez J. C.; Estévez R. J. Transformation ofd-Glucose into 1D-3-Deoxy-3-hydroxymethyl-myo-inositol by Stereocontrolled Intramolecular Henry Reaction. Org. Lett. 2003, 5, 4457–4459. 10.1021/ol035771x. [DOI] [PubMed] [Google Scholar]; c Ballini R.; Petrini M. Nitroalkanes as Key Building Blocks for the Synthesis of Heterocyclic derivatives. ARKIVOC 2009, 9, 195–223. 10.3998/ark.5550190.0010.912. [DOI] [Google Scholar]; d Shen B.; Makley D. M.; Johnston J. N. Umpolung Reactivity in Amide and Peptide Synthesis. Nature 2010, 465, 1027–1032. 10.1038/nature09125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For a recent review:Ballini R.; Palmieri A. Synthetic Procedures for the Preparation of Nitroalkanes. Adv. Synth. Catal. 2018, 360, 2240–2266. and references cited therein 10.1002/adsc.201800163. [DOI] [Google Scholar]
- For selected reviews, see:; a Luzzio F. A. The Henry Reaction: Recent Examples. Tetrahedron 2001, 57, 915–945. 10.1016/s0040-4020(00)00965-0. [DOI] [Google Scholar]; b Palomo C.; Oiarbide M.; Laso A. Recent Advances in the Catalytic Asymmetric Nitroaldol (Henry) Reaction. Eur. J. Org. Chem. 2007, 2561–2574. 10.1002/ejoc.200700021. [DOI] [Google Scholar]; c Alvarez-Casao Y.; Marques-Lopez E.; Herrera R. P. Organocatalytic Enantioselective Henry Reactions. Symmetry 2011, 3, 220–245. 10.3390/sym3020220. [DOI] [Google Scholar]
- For selected recent examples:; a Gildner P. G.; Gietter A. A. S.; Cui D.; Watson D. A. Benzylation of Nitroalkanes Using Copper-Catalyzed Thermal Redox Catalysis: Toward the Facile C-Alkylation of Nitroalkanes. J. Am. Chem. Soc. 2012, 134, 9942–9945. 10.1021/ja304561c. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Yang X.-F.; Yu W.-H.; Ding C.-H.; Ding Q.-P.; Wan S.-L.; Hou X.-L.; Dai L.-X.; Wang P.-J. Palladium-Catalyzed Regio-, Diastereo-, and Enantioselective Allylation of Nitroalkanes with Monosubstituted Allylic Substrates. J. Org. Chem. 2013, 78, 6503–6509. 10.1021/jo400663d. [DOI] [PubMed] [Google Scholar]; c Walvoord R. R.; Kozlowski M. C. Minimizing the Amount of Nitromethane in Palladium-Catalyzed Cross-Coupling with Aryl Halides. J. Org. Chem. 2013, 78, 8859–8864. 10.1021/jo401249y. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Dey C.; Lindstedt E.; Olofsson B. Metal-Free C-Arylation of Nitro Compounds with Diaryliodonium Salts. Org. Lett. 2015, 17, 4554–4557. 10.1021/acs.orglett.5b02270. [DOI] [PubMed] [Google Scholar]
- For a review:Ballini R.; Bosica G.; Fiorini D.; Palmieri A.; Petrini M. Conjugate Additions of Nitroalkanes to Electron-Poor Alkenes: Recent Results. Chem. Rev. 2005, 105, 933–972. 10.1021/cr040602r. [DOI] [PubMed] [Google Scholar]
- For related reviews:; a Berner O. M.; Tedeschi L.; Enders D. Asymmetric Michael additions to nitroalkenes. Eur. J. Org. Chem. 2002, 1877–1894. . [DOI] [Google Scholar]; b Tsogoeva S. B. Recent advances in asymmetric organocatalytic 1,4-conjugate additions. Eur. J. Org. Chem. 2007, 1701–1716. 10.1002/ejoc.200600653. [DOI] [Google Scholar]; c Alonso D.; Baeza A.; Chinchilla R.; Gómez C.; Guillena G.; Pastor I.; Ramón D. Recent Advances in Asymmetric Organocatalyzed Conjugate Additions to Nitroalkenes. Molecules 2017, 22, 895. 10.3390/molecules22060895. [DOI] [PMC free article] [PubMed] [Google Scholar]; and references cited therein
- For selected examples, see:; a Keller E.; Veldman N.; Spek A. L.; Feringa B. L. Catalytic enantioselective Michael addition reactions of α-nitroesters to α,β-unsaturated ketones. Tetrahedron: Asymmetry 1997, 8, 3403–3413. 10.1016/s0957-4166(97)00432-1. [DOI] [Google Scholar]; b Funabashi K.; Saida Y.; Kanai M.; Arai T.; Sasai H.; Shibasaki M. Catalytic Asymmetric Michael Addition of Nitromethane to Enones Controlled by (R)-LPB. Tetrahedron Lett. 1998, 39, 7557–7558. 10.1016/s0040-4039(98)01644-x. [DOI] [Google Scholar]; c Taylor M. S.; Zalatan D. N.; Lerchner A. M.; Jacobsen E. N. Highly Enantioselective Conjugate Additions to α,β-Unsaturated Ketones Catalyzed by a (Salen)Al Complex. J. Am. Chem. Soc. 2005, 127, 1313–1317. 10.1021/ja044999s. [DOI] [PubMed] [Google Scholar]; d Blay G.; Incerti C.; Muñoz M. C.; Pedro J. R. Enantioselective LaIII-pyBOX-Catalyzed Nitro-Michael Addition to (E)-2-Azachalcones. Eur. J. Org. Chem. 2013, 1696–1705. 10.1002/ejoc.201201579. [DOI] [Google Scholar]
- a Baschieri A.; Bernardi L.; Ricci A.; Suresh S.; Adamo M. F. A. Catalytic Asymmetric Conjugate Addition of Nitroalkanes to 4-Nitro-5-styrylisoxazoles. Angew. Chem., Int. Ed. 2009, 48, 9342–9345. 10.1002/anie.200905018. [DOI] [PubMed] [Google Scholar]; b Csákÿ A. G.; de la Herrán G.; Murcia M. C. Conjugate Addition Reactions of Carbon Nucleophiles to Electron-deficient Dienes. Chem. Soc. Rev. 2010, 39, 4080–4102. and references cited therein 10.1039/b924486g. [DOI] [PubMed] [Google Scholar]
- a Chu W.-D.; Zhang L.-F.; Bao X.; Zhao X.-H.; Zeng C.; Du J.-Y.; Zhang G.-B.; Wang F.-X.; Ma X.-Y.; Fan C.-A. Asymmetric Catalytic 1,6-Conjugate Addition/Aromatization of para-Quinone Methides: Enantioselective Introduction of Functionalized Diarylmethine Stereogenic Centers. Angew. Chem., Int. Ed. 2013, 52, 9229–9233. 10.1002/anie.201303928. [DOI] [PubMed] [Google Scholar]; b Gai K.; Fang X.; Li X.; Xu J.; Wu X.; Lin A.; Yao H. Synthesis of Spiro[2.5]octa-4,7-dien-6-one with Consecutive Quaternary Centers via 1,6-Conjugate Addition Induced Dearomatization of para-Quinone Methides. Chem. Commun. 2015, 51, 15831–15834. 10.1039/c5cc06287j. [DOI] [PubMed] [Google Scholar]; c Yuan Z.; Fang X.; Li X.; Wu J.; Yao H.; Lin A. 1,6-Conjugated Addition-Mediated [2+1] Annulation: Approach to Spiro[2.5]octa-4,7-dien-6-one. J. Org. Chem. 2015, 80, 11123–11130. 10.1021/acs.joc.5b01793. [DOI] [PubMed] [Google Scholar]; d Ge L.; Lu X.; Cheng C.; Chen J.; Cao W.; Wu X.; Zhao G. Amide-Phosphonium Salt as Bifunctional Phase Transfer Catalyst for Asymmetric 1,6-Addition of Malonate Esters to para-Quinone Methides. J. Org. Chem. 2016, 81, 9315–9325. 10.1021/acs.joc.6b01906. [DOI] [PubMed] [Google Scholar]; e Zhang X.-Z.; Du J.-Y.; Deng Y.-H.; Chu W.-D.; Yan X.; Yu K.-Y.; Fan C.-A. Spirocyclopropanation Reaction of para-Quinone Methides with Sulfonium Salts: The Synthesis of Spirocyclopropanyl para-Dienones. J. Org. Chem. 2016, 81, 2598–2606. 10.1021/acs.joc.5b02725. [DOI] [PubMed] [Google Scholar]; f He F.-S.; Jin J.-H.; Yang Z.-T.; Yu X.; Fossey J. S.; Deng W.-P. Direct Asymmetric Synthesis of β-Bis-Aryl-α-Amino Acid Esters via Enantioselective Copper-Catalyzed Addition of p-Quinone Methides. ACS Catal. 2016, 6, 652–656. 10.1021/acscatal.5b02619. [DOI] [Google Scholar]; g Zhang X.-Z.; Deng Y.-H.; Gan K.-J.; Yan X.; Yu K.-Y.; Wang F.-X.; Fan C.-A. Tandem Spirocyclopropanation/Rearrangement Reaction of Vinyl p-Quinone Methides with Sulfonium Salts: Synthesis of Spirocyclopentenyl p-Dienones. Org. Lett. 2017, 19, 1752–1755. 10.1021/acs.orglett.7b00516. [DOI] [PubMed] [Google Scholar]; h Yuan Z.; Liu L.; Pan R.; Yao H.; Lin A. Silver-Catalyzed Cascade 1,6-Addition/Cyclization of para-Quinone Methides with Propargyl Malonates: An Approach to Spiro[4.5]deca-6,9-dien-8-ones. J. Org. Chem. 2017, 82, 8743–8751. 10.1021/acs.joc.7b01400. [DOI] [PubMed] [Google Scholar]; i Sun Z.; Sun B.; Kumagai N.; Shibasaki M. Direct Catalytic Asymmetric 1,6-Conjugate Addition of Amides to p-Quinone Methides. Org. Lett. 2018, 20, 3070–3073. 10.1021/acs.orglett.8b01109. [DOI] [PubMed] [Google Scholar]; j Zhang Z.-P.; Xie K.-X.; Yang C.; Li M.; Li X. Asymmetric Synthesis of Dihydrocoumarins through Chiral Phosphoric Acid-Catalyzed Cycloannulation of para-Quinone Methides and Azlactones. J. Org. Chem. 2018, 83, 364–373. 10.1021/acs.joc.7b02750. [DOI] [PubMed] [Google Scholar]; k Zhi Y.; Zhao K.; von Essen C.; Rissanen K.; Enders D. Synthesis of trans-disubstituted-2,3-dihydrobenzofurans by a formal [4 + 1] annulation between para-quinone methides and sulfonium salts. Org. Chem. Front. 2018, 5, 1348–1351. 10.1039/c8qo00008e. [DOI] [Google Scholar]; l Gupta A. K.; Ahamad S.; Vaishanv N. K.; Kant R.; Mohanan K. Base-mediated 1,6-conjugate addition of the Seyferth-Gilbert reagent to para-quinone methides. Org. Biomol. Chem. 2018, 16, 4623–4627. 10.1039/c8ob01017j. [DOI] [PubMed] [Google Scholar]; m Zhang Z.-P.; Chen L.; Li X.; Cheng J.-P. Organocatalytic Asymmetric Sequential 1,6-Addition/Acetalization of 1-Oxotetralin-2-carbaldehyde to ortho-Hydroxyphenyl-Substituted para-Quinone Methides for Synthesis of Spiro-3,4-dihydrocoumarins. J. Org. Chem. 2018, 83, 2714–2724. 10.1021/acs.joc.7b03177. [DOI] [PubMed] [Google Scholar]; n Li W.; Xu X.; Liu Y.; Gao H.; Cheng Y.; Li P. Enantioselective Organocatalytic 1,6-Addition of Azlactones to para-Quinone Methides: An Access to α,α-Disubstituted and β,β-Diaryl-α-amino acid Esters. Org. Lett. 2018, 20, 1142–1145. 10.1021/acs.orglett.8b00072. [DOI] [PubMed] [Google Scholar]; o Liu L.; Yuan Z.; Pan R.; Zeng Y.; Lin A.; Yao H.; Huang Y. 1,6-Conjugated Addition-mediated [4 + 1] Annulation: An Approach to 2,3-Dihydrobenzofurans. Org. Chem. Front. 2018, 5, 623–628. 10.1039/c7qo00846e. [DOI] [Google Scholar]; p Santra S.; Porey A.; Guin J. 1,6-Conjugate Addition of 1,3-Dicarbonyl Compounds to para-Quinone Methides Enabled by Noncovalent N-Heterocyclic Carbene Catalysis. Asian J. Org. Chem. 2018, 7, 477–486. 10.1002/ajoc.201700656. [DOI] [Google Scholar]
- Singh G.; Goswami P.; Vijaya Anand R. Exploring bis-(amino)cyclopropenylidene as a non-covalent Brønsted base catalyst in conjugate addition reactions. Org. Biomol. Chem. 2018, 16, 384–388. 10.1039/c7ob02882b. [DOI] [PubMed] [Google Scholar]
- For selected recent reports:; a Goswami P.; Singh G.; Vijaya Anand R. N-Heterocyclic Carbene Catalyzed 1,6-Conjugate Addition of Me3Si-CN to para-Quinone Methides and Fuchsones: Access to α-Arylated Nitriles. Org. Lett. 2017, 19, 1982–1985. 10.1021/acs.orglett.7b00508. [DOI] [PubMed] [Google Scholar]; b Goswami P.; Sharma S.; Singh G.; Vijaya Anand R. Bis(amino)cyclopropenylidene Catalyzed Rauhut-Currier Reaction between α,β-Unsaturated Carbonyl Compounds and para-Quinone Methides. J. Org. Chem. 2018, 83, 4213–4220. 10.1021/acs.joc.8b00225. [DOI] [PubMed] [Google Scholar]; c Jadhav A. S.; Pankhade Y. A.; Vijaya Anand R. Exploring Gold Catalysis in a 1,6-Conjugate Addition/Domino Electrophilic Cyclization Cascade: Synthesis of Cyclohepta[b]indoles. J. Org. Chem. 2018, 83, 8615–8626. 10.1021/acs.joc.8b00607. [DOI] [PubMed] [Google Scholar]; d Jadhav A. S.; Pankhade Y. A.; Anand R. V. Tandem One-Pot Approach To Access 1,2,3-Triazole-fused Isoindolines through Cu-Catalyzed 1,6-Conjugate Addition of Me3SiN3 to p-Quinone Methides followed by Intramolecular Click Cycloaddition. J. Org. Chem. 2018, 83, 8596–8606. 10.1021/acs.joc.8b00573. [DOI] [PubMed] [Google Scholar]; e Singh G.; Goswami P.; Sharma S.; Anand R. V. A One-Pot Approach to 2,3-Diarylbenzo[b]furans through N-Heterocyclic Carbene-Catalyzed 1,6-Conjugate Addition Followed by Acid Mediated Dehydrative Annulation. J. Org. Chem. 2018, 83, 10546–10554. 10.1021/acs.joc.8b01358. [DOI] [PubMed] [Google Scholar]; f Jadhav A. S.; Pankhade Y. A.; Hazra R.; Anand R. V. 1,6-Hydroolefination and Cascade Cyclization of p-Quinone Methides with Styrenes: Total Synthesis of (±)-Isopaucifloral F. J. Org. Chem. 2018, 83, 10107–10119. 10.1021/acs.joc.8b01401. [DOI] [PubMed] [Google Scholar]
- a Yoshida J.Flash Chemistry. In Fast Organic Synthesis in Microsystems; Wiley-Blackwell, 2008. [Google Scholar]; b Ehrfeld W.; Hessel V.; Löwe H.. Microreactors; Wiley-VCH Verlag GmbH: Weinheim, 2010. [Google Scholar]; c Wiles C.; Watts P.. Micro Reaction Technology in Organic Synthesis; CRC Press: Boca Raton, FL, 2011. [Google Scholar]
- For selected reviews, see:; a Watts P.; Haswell S. J. The Application of Micro Reactors for Organic Synthesis. Chem. Soc. Rev. 2005, 34, 235–246. 10.1039/b313866f. [DOI] [PubMed] [Google Scholar]; b Geyer K.; Codée J. D. C.; Seeberger P. H. Microreactors as Tools for Synthetic Chemists-The Chemists’ Round-Bottomed Flask of the 21st Century?. Chem.—Eur. J. 2006, 12, 8434–8442. 10.1002/chem.200600596. [DOI] [PubMed] [Google Scholar]; c Mason B. P.; Price K. E.; Steinbacher J. L.; Bogdan A. R.; McQuade D. T. Greener Approaches to Organic Synthesis Using Microreactor Technology. Chem. Rev. 2007, 107, 2300–2318. 10.1021/cr050944c. [DOI] [PubMed] [Google Scholar]; d Razzaq T.; Kappe C. O. Continuous Flow Organic Synthesis under High-Temperature/Pressure Conditions. Chem.—Asian J. 2010, 5, 1274–1289. 10.1002/asia.201000010. [DOI] [PubMed] [Google Scholar]; e Wegner J.; Ceylan S.; Kirschning A. Flow Chemistry - A Key Enabling Technology for (Multistep) Organic Synthesis. Adv. Synth. Catal. 2012, 354, 17–57. 10.1002/adsc.201100584. [DOI] [Google Scholar]; f Gemoets H. P. L.; Su Y.; Shang M.; Hessel V.; Luque R.; Noël T. Liquid Phase Oxidation Chemistry in Continuous Flow Microreactors. Chem. Soc. Rev. 2016, 45, 83–117. 10.1039/c5cs00447k. [DOI] [PubMed] [Google Scholar]; g Movsisyan M.; Delbeke E. I. P.; Berton J. K. E. T.; Battilocchio C.; Ley S. V.; Stevens C. V. Taming Hazardous Chemistry by Continuous Flow Technology. Chem. Soc. Rev. 2016, 45, 4892–4928. 10.1039/c5cs00902b. [DOI] [PubMed] [Google Scholar]; h Cambié D.; Bottecchia C.; Straathof N. J. W.; Hessel V.; Noël T. Applications of Continuous Flow Photochemistry in Organic Synthesis, Material Science, and Water Treatment. Chem. Rev. 2016, 116, 10276–10341. 10.1021/acs.chemrev.5b00707. [DOI] [PubMed] [Google Scholar]; i Plutschack M. B.; Pieber B.; Gilmore K.; Seeberger P. H. The Hitchhiker’s Guide to Flow Chemistry. Chem. Rev. 2017, 117, 11796–11893. 10.1021/acs.chemrev.7b00183. [DOI] [PubMed] [Google Scholar]
- For selected recent reviews:; a Webb D.; Jamison T. F. Continuous Flow Multi-step Organic Synthesis. Chem. Sci. 2010, 1, 675–680. 10.1039/c0sc00381f. [DOI] [Google Scholar]; b Pastre J. C.; Browne D. L.; Ley S. V. Flow Chemistry Syntheses of Natural Products. Chem. Soc. Rev. 2013, 42, 8849–8869. 10.1039/c3cs60246j. [DOI] [PubMed] [Google Scholar]; c Gutmann B.; Cantillo D.; Kappe C. O. Continuous Flow Technology-A Tool for the Safe Manufacturing of Active Pharmaceutical Ingredients. Angew. Chem., Int. Ed. 2015, 54, 6688–6728. 10.1002/anie.201409318. [DOI] [PubMed] [Google Scholar]; d Porta R.; Benaglia M.; Puglisi A. Flow Chemistry: Recent Developments in the Synthesis of Pharmaceutical Products. Org. Process Res. Dev. 2016, 20, 2–25. 10.1021/acs.oprd.5b00325. [DOI] [Google Scholar]; e Britton J.; Raston C. L. Multi-step Continuous Flow Synthesis. Chem. Soc. Rev. 2017, 46, 1250–1271. 10.1039/c6cs00830e. [DOI] [PubMed] [Google Scholar]
- For recent reviews:; a Mak X. Y.; Laurino P.; Seeberger P. H. Asymmetric Reactions in Continuous Flow. Beilstein J. Org. Chem. 2009, 5, 19. 10.3762/bjoc.5.19. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Atodiresei I.; Vila C.; Rueping M. Asymmetric Organocatalysis in Continuous Flow: Opportunities for Impacting Industrial Catalysis. ACS Catal. 2015, 5, 1972–1985. 10.1021/acscatal.5b00002. [DOI] [Google Scholar]
- a Jadhav A. S.; Anand R. V. 1,6-Conjugate Addition of Zinc Alkyls to para-Quinone Methides in a Continuous Flow Microreactor. Org. Biomol. Chem. 2017, 15, 56–60. 10.1039/c6ob02277d. [DOI] [PubMed] [Google Scholar]; b Jadhav A. S.; Anand R. V. Triflic Acid Catalyzed 1,6-Conjugate Addition of Thiols to p -Quinone Methides under Continuous Flow Conditions. Eur. J. Org. Chem. 2017, 3716–3721. 10.1002/ejoc.201700587. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.