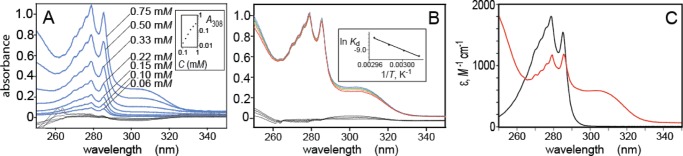
Figure 3.
UV absorption spectra demonstrating the reversible complexation of p-cresol with equimolar dodecylguanidine in hexane. Spectra were obtained as a function of total concentration of p-cresol (A, in which all seven spectra were measured at 58 °C; the inset shows a log–log plot of A308 vs total concentration); or as a function of temperature (B, in which all eight spectra were measured with 0.50 mM total concentrations of p-cresol and dodecylguanidine; the inset shows a van’t Hoff plot). Temperatures in (B) are indicated by trace colors: blue, 58 °C; green, 60 °C; orange, 62 °C; red, 64 °C. Two nearly superimposed traces of each color are plotted, measured during up-then-down temperature steps. Spectral traces in black (overlapping curves near 0 absorbance) in (A) and (B) represent the residuals after subtracting the best-fitted linear combination of the spectra of free p-cresol and complexed p-cresol/dodecylguanidine (C). The contribution of free p-cresol was computed as the measured extinction-coefficient spectrum of pure p-cresol in hexane (C, black trace), multiplied by its measured total concentration and the best-fit mole fraction for it. These 11 mole fractions of free p-cresol, 1 for each of the 11 measured spectra, were calculated as 1 – Xi, where the values of Xi were 11 of the 512 adjustable parameters during the least-squares fit. These Xi were the mole fractions of the p-cresol present as a 1:1 complex with dodecylguanidine. All 501 values of the extinction-coefficient spectrum of this 1:1 complex (C, red trace) were also optimized during the fit. See Supporting Information for further details.