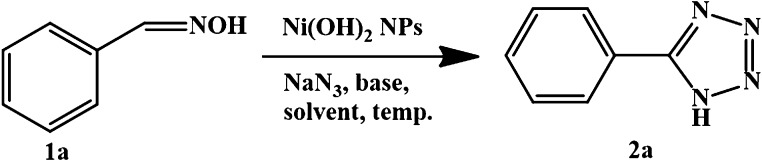
Table 1. Optimization of Reaction Conditionsa.
| entry | Cat. (mol %) | solvent | base | temp (°C) | yield (%)b |
|---|---|---|---|---|---|
| 1 | K2CO3 | 120 | |||
| 2 | 4.32 | DMF | K2CO3 | 120 | 30 |
| 3 | 4.32 | p-xylene | K2CO3 | 120 | 83 |
| 4 | 4.32 | toluene | K2CO3 | 110 | 56 |
| 5 | 4.32 | dioxane | K2CO3 | 105 | 22 |
| 6 | 4.32 | K2CO3 | 120 | 52 | |
| 7 | 4.32 | water | K2CO3 | 100 | 98 |
| 8 | 4.32 | water | K2CO3 | 65 | 38 |
| 9 | 4.32 | water | K2CO3 | rt | |
| 10 | 4.32 | water | NaHCO3 | 100 | 68 |
| 11 | 4.32 | water | Cs2CO3 | 100 | 32 |
| 12 | 4.32 | water | K3PO4 | 100 | 23 |
| 13 | 4.32 | water | Na2CO3 | 100 | 72 |
| 14 | 8.6 | water | K2CO3 | 100 | 93 |
1a (1.0 mmol), NaN3 (1.5 mmol), base (3.0 mmol), solvent (3.0 mL), Ni(OH)2 NPs, 10 h.
Yields are obtained by gas chromatography.