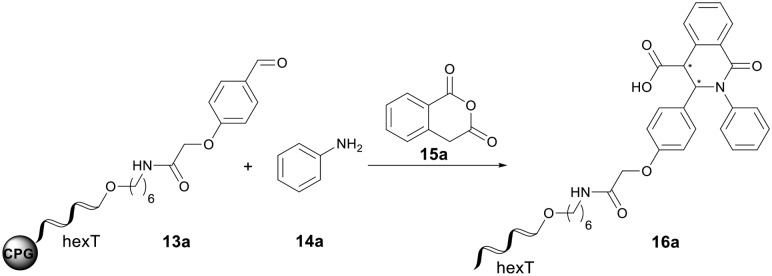
Table 1. Optimization of the Yb(OTf)3-mediated Castagnoli–Cushman reaction to the CPG-bound hexT-isoquinolonic acid conjugate 16a a .
| |||||
| Entry | Yb(OTf)3 [equiv.] | t 1 [h] | t 2 [h] | Solvent | Conversion b [%] |
| 1 c | 100 | 4 | 2 | CH2Cl2 | <5 |
| 2 | 100 | 4 | 2 | CH2Cl2 | 72 |
| 3 | 100 | 4 | 1 | CH2Cl2 | 73 |
| 4 | 100 | 4 | 0.5 | CH2Cl2 | 68 |
| 5 | 100 | 3 | 1 | CH2Cl2 | 69 |
| 6 | 100 | 2 | 1 | CH2Cl2 | 68 |
| 7 | 100 | 1 | 1 | CH2Cl2 | 65 |
| 8 | 100 | 16 | 1 | CH2Cl2 | 73 |
| 9 d | 100 | 4 | 1 | CH2Cl2 | 7 |
| 10 | 50 | 4 | 1 | CH2Cl2 | 70 |
| 11 | 25 | 4 | 1 | CH2Cl2 | 54 |
| 12 | 0 | 4 | 1 | CH2Cl2 | 38 |
| 13 e | 50 | 4 | 1 | CH2Cl2 | 65 |
| 14 f | 50 | 4 | 1 | CH2Cl2 | 63 |
| 15 g | 50 | 4 | 1 | CH2Cl2 | 51 |
| 16 | 50 | 4 | 1 | THF | 56 |
| 17 | 50 | 4 | 1 | DCE | 58 |
| 18 | 50 | 4 | 1 | ACN | 52 |
| 19 | 50 | 4 | 1 | MeOH | 68 |
| 20 | 50 | 4 | 1 | Toluene | 50 |
| 21 | 50 | 4 | 1 | 1,4-Dioxane | 52 |
| 22 | 50 | 4 | 1 | DMF | 31 |
| 23 | 50 | 4 | 1 | EtOAc | 40 |
aCPG-bound oligonucleotide conjugate 13a (20 nmol) and aniline 14a (500 equiv., 10 μmol) in 36 μL of solvent/triethyl orthoformate (2 : 1) at ambient temperature for t1, then Yb(OTf)3 (X equiv., 1 μmol) and anhydride 15a (500 equiv., 10 μmol) both suspended in 30 μL of solvent, at ambient temperature for t2. Afterwards, with AMA (30% aqueous ammonia/40% aqueous methylamine, 1 : 1 (vol/vol)) at ambient temperature for 0.5 h.
bDetermined by analytical RP-HPLC analysis.
cWashing of the CPG-bound hexT-conjugate after imine formation.
dImine formation performed without triethyl orthoformate.
e1000 equiv. of aniline 14a and anhydride 15 were used.
f1500 equiv. of aniline 14a and anhydride 15a were used.
g2000 equiv. of aniline 14a and anhydride 15 were used.
